Editor’s Choice

Competition for publication in Diabetologia continues to grow, and less than 20% of papers are accepted. Of all the high-quality papers I want to draw your attention to articles that I think stand out in some regard and are very interesting. The articles are summarised here. Our publisher, Springer, has kindly made the full text of each of these papers freely available. I hope you enjoy reading them! These will be featured ‘up front’ in the print issue and here on our website. Hindrik Mulder, Editor
Phenotype‑based targeted treatment of SGLT2 inhibitors and GLP‑1 receptor agonists in type 2 diabetes – published online 22/02/2024

Pedro Cardoso, Katie G. Young, Anand T. N. Nair, Rhian Hopkins, Andrew P. McGovern, Eram Haider, Piyumanga Karunaratne, Louise Donnelly, Bilal A. Mateen, Naveed Sattar, Rury R. Holman, Jack Bowden, Andrew T. Hattersley, Ewan R. Pearson, Angus G. Jones, Beverley M. Shields, Trevelyan J. McKinley, John M. Dennis, on behalf of the MASTERMIND consortium
A central aim of type 2 diabetes precision medicine is to accurately target specific drug treatments to the individuals most likely to benefit from them. In this issue, Cardoso et al (https://doi.org/10.1007/s00125-024-06099-3) apply cutting-edge Bayesian causal forest models to develop and validate a model to predict differences in the glycaemic efficacy of glucagon-like peptide-1 (GLP-1) receptor agonists and sodium−glucose cotransporter 2 (SGLT2) inhibitors for individuals based on their routine clinical characteristics. Using large-scale UK routine clinical data (n=46,394), the authors identify robust and clinically relevant differences in glycaemic response for many individuals. Sex is identified as a major treatment response modifier, with greater glycaemic efficacy of GLP-1 receptor agonists in females, a finding confirmed in independent trial data. Beyond glycaemia, targeting of both therapies based on predicted HbA1c response was associated with improved short-term tolerability and lower longer-term risk of new-onset microvascular complications. The authors conclude that the use of routine clinical features for type 2 diabetes treatment selection could support low-cost precision medicine worldwide.
Safety, tolerability and immunogenicity of PRV‑101, a multivalent vaccine targeting coxsackie B viruses (CVBs) associated with type 1 diabetes: a double‑blind randomised placebo‑controlled Phase I trial – published online 19/02/2024

Heikki Hyöty, Susanna Kääriäinen, Jutta E. Laiho, Gail M. Comer, Wei Tian, Taina Härkönen, Jussi P. Lehtonen, Sami Oikarinen, Leena Puustinen, Michele Snyder, Francisco León, Mika Scheinin, Mikael Knip, Miguel Sanjuan
Coxsackie B virus (CVB) infections usually cause mild common cold-type symptoms but may sometimes lead to severe life-threatening conditions. Recent epidemiological, mechanistic and preclinical studies point to CVB infections as the main environmental factor that sets into motion the autoimmune process that eliminates pancreatic beta cells in genetically susceptible individuals, triggering type 1 diabetes. There are no approved vaccines against CVB, and PRV-101, a formalin-inactivated vaccine including CVB serotypes 1–5, is the first one to progress to human testing. In this issue, Hyöty et al (https://doi.org/10.1007/s00125-024-06092-w) report that PRV-101 was well tolerated by participants in a double-blind randomised placebo-controlled Phase I trial. PRV-101 induced long-lasting, high serum concentrations of neutralising antibodies against all five CVB serotypes included in the vaccine. The authors conclude that the results of this first-in-human trial support the future development of PRV-101, which has the potential to be the first vaccine to prevent CVB infections, and could therefore potentially decrease the global incidence of type 1 diabetes.
Harnessing the power of proteomics in precision diabetes medicine – published online 12/02/2024

Nigel Kurgan, Jeppe Kjærgaard Larsen, Atul S. Deshmukh
High-throughput and robust proteomic technologies have developed rapidly over the past decade. In this issue, Kurgan et al (https://doi.org/10.1007/s00125-024-06097-5) propel proteomics to the forefront of advancing precision diabetes medicine. They summarise recent advances in cutting-edge MS-based and affinity proteomic technologies and describe how they can be applied to improve accuracy in the prevention, diagnosis, prognosis and treatment of diabetes. The authors present a compelling case for proteomics in identifying predictive protein panels for type 2 diabetes subtyping and constructing clinical prediction models. Moreover, they underscore the significance of integrating proteomics with multimodal data, including genomics (proteogenomics) and clinical information, to deepen our understanding of the heterogeneity in type 2 diabetes aetiology and treatment responsiveness. The authors conclude with a call for action formed on advancing proteomics technologies, benchmarking their performance and standardising technologies across sites. The figures from this review are available as a downloadable slideset.
Keeping pace: the primary cilium as the conducting baton of the islet – published online 14/02/2024

Olof Idevall‑Hagren, Ceren Incedal Nilsson, Gonzalo Sanchez
The cilium is probably the most elusive of the cellular organelles and its functions are only just beginning to be explored and understood. The fact that it is the only organelle exposed to the extracellular milieu and its surface is packed with receptors clearly points to its involvement as a sensory platform, including in pancreatic islets. New research on the basic structural and functional features of the cilium in beta cells is converging with genome-wide association studies to support key roles of the cilium in both cell and organ-level physiology, including regulation of cell polarity, metabolism, and production and secretion of insulin. In this issue, Idevall-Hagren et al (https://doi.org/10.1007/s00125-024-06096-6) discuss the latest findings and the challenges ahead with an eye on the potential for developing strategies to steer ciliary signalling to produce therapeutical impact. The figures from this review are available as a downloadable slideset.
Feasibility of prevention of type 2 diabetes in low‑ and middle‑income countries – published online 15/02/2024

Andre P Kengne, Ambady Ramachandran
The population of people with type 2 diabetes in low- and middle-income countries (LMICs) is huge and fast growing, calling for efforts to prevent further increases, while improving the detection and management of people with the condition. In this issue, Kengne and Ramachandran (https://doi.org/10.1007/s00125-023-06085-1) highlight the practical challenges in preventing type 2 diabetes and discuss the steps involved in the implementation of effective type 2 diabetes prevention programmes in LMICs. Existing evidence on type 2 diabetes prevention from both efficacy and implementation studies originates mostly from high income countries and, in LMICs, is dominated by studies from China and India. Challenges and barriers to implementing diabetes prevention programmes in LMICs include individual-, societal- and healthcare support-level factors. Going forward, steps in effectively implementing diabetes prevention programmes in LMICs while addressing the existing challenges should include awareness creation, risk screening of individuals, planning the programmes, and outcome evaluation. The authors conclude that non-invasive risk factor-based screening and information technology-based implementation strategies hold promise. The figure from this review is available as a downloadable slide.
Skeletal muscle TET3 promotes insulin resistance through destabilisation of PGC-1α – published online 13/01/2024

Beibei Liu, Di Xie, Xinmei Huang, Sungho Jin, Yangyang Dai, Xiaoli Sun, Da Li, Anton M. Bennett, Sabrina Diano, Yingqun Huang
Skeletal muscle insulin resistance is a critical component of the pathogenesis of type 2 diabetes. Decreased expression of PGC-1α is among the many mechanisms implicated in insulin resistance; however, how this dysregulation occurs is yet to be elucidated. In this issue Liu, Xie, Huang et al (https://doi.org/10.1007/s00125-023-06073-5) report increased expression of ten-eleven translocation 3 (TET3) in skeletal muscle of individuals with type 2 diabetes as compared with individuals without diabetes. They show that TET3 interacts with and reduces the abundance of PGC-1α in myocytes. Specifically, TET3 was found to form protein complexes with PGC-1α, preventing its phosphorylation on sites known to promote protein stability and activity. It is proposed that this results in decreased mitochondrial respiration and insulin sensitivity in myocytes. Consistent with this theory, the authors showed that mice with skeletal muscle-specific TET3 deficiency exhibited increased PGC-1α abundance, and enhanced muscle and whole-body insulin sensitivity. Moreover, these animals remained insulin sensitive under high-fat diet challenge. The authors conclude that these findings hold promise for developing novel, TET3-targeting therapeutic agents for insulin resistance and type 2 diabetes.
Genetic engineering of regulatory T cells for treatment of autoimmune disorders including type 1 diabetes – published online 18/01/2024

Karoliina Tuomela, Megan K. Levings
Advanced genetic engineering approaches are transforming cell therapies across a number of fields, from cancer to autoimmunity. In this issue, Tuomela and Levings (https://doi.org/10.1007/s00125-023-06076-2) discuss the role and potential of immunosuppressive regulatory T cells (Tregs) in type 1 diabetes, focusing on the opportunities presented by the genetic engineering of these cells in this context. The authors highlight that although Tregs have demonstrated excellent safety in clinical trials, there has been a clear need for improved efficacy and consideration for the unique challenges in type 1 diabetes. Fortunately, genetic engineering has led to significant advancements in the areas of Treg manufacture, antigen specificity and in vivo survival, leading to promising pre-clinical results. As a result, engineered Treg therapies are on the verge of entry into clinical trial for the treatment type 1 diabetes. Nevertheless, the authors conclude that questions remain regarding the optimal strategy for designing Tregs that effectively suppress immune responses in the pancreatic islet environment. The figure from this review is available as a downloadable slide.
Remission of type 2 diabetes: always more questions, but enough answers for action – published online 08/01/2024

Amy Rothberg, Michael Lean, Blandine Laferrère
The concept of type 2 diabetes remission is gaining wide public and professional attention. In this issue, Rothberg, Lean and Laferrère (https://doi.org/10.1007/s00125-023-06069-1) discuss how substantial and sustained intentional weight loss can result in durable remission, especially if implemented early in the onset of the disease, preferably at the stage of prediabetes (defined in Europe, Australasia and Canada [and most of the world] as HbA1c ≥42 mmol/mol and <48 mmol/mol [≥6.0% and <6.5%], and in the USA as HbA1c ≥39 mmol/mol and <48 mmol/mol [≥5.7% and <6.5%]). Effective weight management also improves all features of the metabolic syndrome and reduces complications. The authors highlight that, although newer medications, such as glucagon-like peptide-1 receptor agonists and sodium–glucose cotransporter-2 receptor inhibitors, represent a formidable leap forward in the treatment of type 2 diabetes and associated obesity, and for the prevention of cardiovascular complications, their cost and side effects are still prohibitive for many. They conclude that affordable intensive lifestyle management should be provided as first-line therapy for the treatment of type 2 diabetes. According to the authors, the greatest research challenges are to improve adherence to a healthy lifestyle and long-term weight loss maintenance, and to define cost-effective approaches tailored to the preferences and needs of people living with type 2 diabetes. The figure from this review is available as a downloadable slide.
Epidemiology of heart failure in diabetes: a disease in disguise – published online 09/02/2024

Anna G. Hoek, Elisa Dal Canto, Eva Wenker, Navin Bindraban, M. Louis Handoko, Petra J. M. Elders, Joline W. J. Beulens
Heart failure (HF) and type 2 diabetes are closely linked, and concomitantly pose an increased risk of morbidity and mortality. In this issue, Hoek et al (https://doi.org/10.1007/s00125-023-06068-2) present a comprehensive review of the epidemiology of HF in people with type 2 diabetes using both a narrative and systematic review approach. In their systematic review/meta-analysis, the authors reveal that there is a higher prevalence of left ventricular diastolic dysfunction (LVDD; 43%) and HF with preserved ejection fraction (HFpEF; 17%) in type 2 diabetes, as compared with left ventricular systolic dysfunction (LVSD; 6%) and HF with reduced ejection fraction (HFrEF; 7%). Furthermore, HFpEF incidence (7%) was shown to surpass HFrEF incidence (4%), emphasising the predominance of LVDD/HFpEF in type 2 diabetes. For LVDD, when assessed by grade (grade I, II or II) or by classification (indeterminate vs definitive), grade I and indeterminate LVDD were highly prevalent; this indicates that there is a large pre-clinical group with early LVDD that could be targeted for early disease detection, to reduce disease burden. The authors conclude that there is a need for easily accessible and reliable tools for diagnosing HF. They outline how the introduction of uniform and accessible guidelines for diagnosing HF (published by the European Society of Cardiology [ESC] in 2021) and the use of sodium–glucose cotransporter 2 inhibitors may lead to more effective treatment of HF in type 2 diabetes. The figures from this review are available as a downloadable slideset.
GLP‑1 metabolite GLP‑1(9–36) is a systemic inhibitor of mouse and human pancreatic islet glucagon secretion – 21/12/2023

Nikhil R. Gandasi, Rui Gao, Lakshmi Kothegala, Abigail Pearce, Cristiano Santos, Samuel Acreman, Davide Basco, Anna Benrick, Margarita V. Chibalina, Anne Clark, Claudia Guida, Matthew Harris, Paul R. V. Johnson, Jakob G. Knudsen, Jinfang Ma, Caroline Miranda, Makoto Shigeto, Andrei I. Tarasov, Ho Yan Yeung, Bernard Thorens, Ingrid W. Asterholm, Quan Zhang, Reshma Ramracheya, Graham Ladds, Patrik Rorsman
The incretin hormone glucagon-like peptide 1 (GLP-1) stimulates insulin secretion and inhibits glucagon secretion, with both effects contributing to its blood glucose-lowering effect. GLP-1 is secreted in the gut as GLP-1(7–36), which is rapidly degraded to GLP-1(9–36) and which was previously believed to be biologically inactive. In this issue, Gandasi et al (https://doi.org/10.1007/s00125-023-06060-w) show that GLP-1(9–36), while lacking insulin releasing capacity, strongly and potently inhibits glucagon secretion. They demonstrate that circulating GLP-1(9–36) functions as a systemic inhibitor of glucagon secretion and that this effect is impaired in type 2 diabetes. The capacity of GLP-1(9–36) to inhibit glucagon secretion is prevented by glucagon receptor antagonists and the marked increase in circulating glucagon after administration of such compounds may (in part) be mediated by the removal of the glucagonostatic effects of GLP-1(9–36). The authors suggest that GLP-1(9–36) is more than just a biologically inactive metabolite and that it has many properties that qualify it as a new systemic glucagonostatic hormone.
Cardiovascular and mortality outcomes with GLP-1 receptor agonists vs other glucose-lowering drugs in individuals with NAFLD and type 2 diabetes: a large population-based matched cohort study – published online 20/12/2023

Arunkumar Krishnan, Carolin V. Schneider, Yousaf Hadi, Diptasree Mukherjee, Bandar AlShehri,·Saleh A Alqahtani
Current evidence highlights a strong association between CVD and non-alcoholic fatty liver disease (NAFLD). The presence of NAFLD in association with type 2 diabetes worsens the metabolic profile and exacerbates the risk of CVD. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have been reported to effectively reduce the incidence of major adverse cardiovascular events (MACE) in individuals with type 2 diabetes or NAFLD. However, the specific impact of GLP-1RAs on cardiovascular risk in individuals with both NAFLD and concurrent type 2 diabetes remains unclear. In this issue, Krishnan et al (https://doi.org/10.1007/s00125-023-06057-5) reveal that, among people with both NAFLD and type 2 diabetes, new GLP-1RA users experienced a lower incidence of MACE, cerebrovascular events, heart failure and mortality than those taking other glucose-lowering medications, with outcomes comparable to those in users of sodium-glucose cotransporter-2 (SGLT2) inhibitors. The findings suggest that early initiation of GLP-1RAs or SGLT2 inhibitors in individuals with NAFLD and type 2 diabetes has potential cardioprotective benefits. The authors conclude that regular cardiovascular risk assessments and prompt incorporation of either of these drugs are crucial for mitigating risks in these individuals. In addition, comparison of the effects of these two drugs may allow drug selection to be personalised based on individual patient needs.
Type 2 diabetes and succinate: unmasking an age‑old molecule – published online 05/01/2024

Sonia Fernandez‑Veledo, Anna Marsal‑Beltran, Joan Vendrell
Succinate, traditionally associated with the tricarboxylic acid (TCA) cycle, has now emerged as a key player in cellular signalling via its receptor, succinate receptor 1 (SUCNR1). In this issue, Fernández-Veledo et al (https://doi.org/10.1007/s00125-023-06063-7) explore the role of succinate in diabetes, shedding light on its evolution from a conventional intracellular metabolite to a potent extracellular signalling molecule. Succinate’s multifaceted nature, with origins in both mitochondria and the microbiome, opens doors to understanding and managing complex metabolic diseases such as diabetes. Recent insights into succinate’s biology highlight its potential as both a biomarker in and a therapeutic target for managing diabetes and its related complications, presenting exciting prospects for future research and clinical interventions. The figure from this review is available as a downloadable slide.
The impact of taxing sugar-sweetened beverages on diabetes: a critical review – published online 04/01/2024

José L. Peñalvo
The global prevalence of type 2 diabetes is rapidly rising, posing significant health and economic challenges, alongside evident disparities. The obesity epidemic exacerbates this issue, with sugar-sweetened beverages (SSBs) contributing to an excess intake of sugar and increased diabetes risk. In this issue, José Peñalvo (https://doi.org/10.1007/s00125-023-06064-6) discusses the implementation of SSB taxes as a promising public-health strategy to curb consumption of these beverages and alleviate the burden of type 2 diabetes. The author highlights how research indicates that such taxes lead to higher prices and reduced consumption of SSBs, particularly among lower socioeconomic groups, potentially reducing health inequalities. Ongoing tax schemes demonstrate positive effects on consumption patterns, with modelling studies predicting health benefits by preventing type 2 diabetes and related conditions. However, empirical evaluation of the impact of SSB taxes remains limited. Peñalvo concludes that continued research and tailored policies, coupled with complementary approaches to reduce diabetes and its risk factors, are crucial to effectively address the global type 2 diabetes crisis. The figure from this review is available as a downloadable slide.
Advances and challenges in measuring hepatic glucose uptake with FDG PET: implications for diabetes research – published online 15/12/2023

Jeremy Basset‑Sagarminaga, Tineke van de Weijer, Patricia Iozzo, Patrick Schrauwen, Vera Schrauwen‑Hinderling
The liver, a pivotal organ in maintaining glucose balance, has emerged as a focal point in the quest to understand the pathogenesis of type 2 diabetes. Hepatic glucose uptake (HGU), a critical aspect of liver metabolism, can be measured using positron emission tomography (PET), but there are unique challenges related to the physiology and metabolic complexity of the liver. In this issue, Basset-Sagarminaga et al (https://doi.org/10.1007/s00125-023-06055-7) provide a comprehensive perspective on the array of protocols available for the measurement of HGU using [18F]-2-fluoro-2-deoxy-d-glucose (FDG) PET and delve into the current state of knowledge concerning HGU and its dysregulation in the context of type 2 diabetes. The authors conclude that FDG PET techniques hold the potential to reshape our understanding of metabolic diseases and advance therapeutic strategies. The figures from this review are available as a downloadable slideset
Disrupted hypothalamic transcriptomics and proteomics in a mouse model of type 2 diabetes exposed to recurrent hypoglycaemia – published online 28/11/2023

Judit Castillo‑Armengol, Flavia Marzetta, Ana Rodriguez Sanchez‑Archidona, Christian Fledelius, Mark Evans, Alison McNeilly, Rory J. McCrimmon, Mark Ibberson, Bernard Thorens
Repeated insulin-induced hypoglycaemia in individuals with diabetes progressively leads to defective counterregulation to restore normoglycaemia, particularly resulting in a decrease in glucagon secretion. This defect is thought to be caused by impaired hypoglycaemia sensing by hypothalamic neurons, although the precise mechanisms are mostly unknown. In this issue, Castillo-Armengol et al (https://doi.org/10.1007/s00125-023-06043-x) report findings from a study in which they developed a mouse model of type 2 diabetes with defective glucagon secretion caused by repeated hypoglycaemic episodes. Using this mouse model, they analysed hypothalamic gene expression via single-nuclei RNA sequencing and performed proteomic analysis of hypothalamic synaptosomal fractions. The authors show that repeated exposure to hypoglycaemia induces changes in neurons, oligodendrocytes and astrocytes that point to reduced sensing of hypoglycaemia, decreased activity of tripartite synapses, and impaired myelination. They also demonstrate increased signs of neurodegeneration with a high propensity for amyloid beta production in these cells. In summary, the authors state that the findings from this study help to define the hypothalamic causes of defective counterregulation and may lead to measures aimed at preventing hypoglycaemic episodes in insulin-treated individuals with diabetes.
Impact of metformin and Dysosmobacter welbionis on diet‑induced obesity and diabetes: from clinical observation to preclinical intervention – published online 28/10/2023

Emilie Moens de Hase, Audrey M. Neyrinck, Julie Rodriguez, Miriam Cnop, Nicolas Paquot, Jean‑Paul Thissen, Yining Xu, Ana Beloqui, Laure B. Bindels, Nathalie M. Delzenne, Matthias Van Hul, Patrice D. Cani
Dysosmobacter welbionis is a commensal intestinal bacterium, the abundance of which is inversely associated with HbA1c in individuals with obesity and type 2 diabetes. In this issue, Moens de Hase et al (https://doi.org/10.1007/s00125-023-06032-0) report that individuals who respond positively to prebiotic treatment, marked by a reduction in BMI after 3 months of intervention, had higher levels of D. welbionis at baseline compared with non-responders. Furthermore, participants treated with metformin exhibited significantly increased levels of this bacterium, while it was inversely linked to fasting blood glucose levels. The authors also show that, mechanistically, D. welbionis appeared to boost the secretion of key hormones, like glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). In mice, D. welbionis treatment not only curtailed weight gain and improved glucose tolerance but also outperformed metformin. The authors conclude that these findings hint at the pivotal role that D. welbionis might play in shaping our metabolic health, with the evidence suggesting that the abundance of D. welbionis is influenced by metformin treatment and associated with prebiotic response and glucose metabolism in individuals with obesity and diabetes. They conclude that their findings may have implications for the development of personalised approaches for the treatment of obesity and diabetes.
Mineralocorticoid receptor overactivation: targeting systemic impact with non‑steroidal mineralocorticoid receptor antagonists – published online 21/12/2023

Gianluigi Savarese, Felix Lindberg, Gerasimos Filippatos, Javed Butler, Stefan D. Anker
Overactivation of the mineralocorticoid receptor (MR) has pathophysiological implications in multiple organ systems. In this issue, Savarese and Lindberg et al (https://doi.org/10.1007/s00125-023-06031-1) provide a comprehensive review of the role of MR overactivation in cardiac and renal disease, and summarise the evidence related to old and new methods of pharmacologically targeting the MR. The authors describe how traditional steroidal MR antagonists (MRA) are a cornerstone of therapy in heart failure but remain underused due to real and perceived risks of side effects, particularly in patients with renal failure and/or at risk of hyperkalaemia. Novel non-steroidal MRA have distinct properties that might enable inhibition of the MR with an improved safety profile. The authors highlight how the Phase III programme on one such novel non-steroidal MRA, finerenone, demonstrated improved renal and cardiovascular outcomes in patients with diabetic kidney disease, potentially setting the stage for a new paradigm in targeting MR overactivation. The figures from this review are available as a downloadable slideset.
A narrative commentary about interoperability in medical devices and data used in diabetes therapy from an academic EU/UK/US perspective – published online 02/12/2023

Johan Jendle, Peter Adolfsson, Pratik Choudhary, Klemen Dovc, Alexander Fleming, David C. Klonoff, Julia K. Mader, Nick Oliver, Jennifer L. Sherr, Jan Šoupal, Lutz Heinemann
People living with diabetes often use a large variety of medical devices to assist their day-to-day diabetes management. In this issue, Jendle et al (https://doi.org/10.1007/s00125-023-06049-5) summarise the current understanding of interoperability in medical devices and data used in diabetes therapy. The authors highlight that a critical aspect of interoperability is how diabetes technology, such as systems for integrated continuous glucose monitoring and automated insulin delivery (AID) systems, communicate with each other. Furthermore, they state that how the data generated by these devices are not only effectively downloaded, integrated and presented, but also effectively and safely used by the individuals living with diabetes and their healthcare providers are also important aspects. As well as the practical challenges, the authors report that connected devices must also adhere to regulatory and legal frameworks, with key issues relating to data ownership and the integrity of connected devices. The authors conclude that an open and transparent standard for data handling remains to be established and only when data can be assessed in a standardised manner can the data generated be integrated into electronic medical records. The figures from this review are available as a downloadable slideset.
Diabetes and artificial intelligence beyond the closed loop: a review of the landscape, promise and challenges – published online 18/11/2023

Scott C. Mackenzie, Chris A. R. Sainsbury, Deborah J. Wake
The rise of artificial intelligence (AI) has brought both promise and apprehension across numerous industries, and healthcare is no exception. In the context of diabetes care, current AI technologies predominantly target type 1 diabetes, representing just the tip of the iceberg in terms of potential. In this review, Mackenzie et al (https://doi.org/10.1007/s00125-023-06038-8) provide a comprehensive perspective on how AI stands poised to revolutionise the entire spectrum of diabetes care. This transformation encompasses empowering self-management, delivering personalised educational support, and leveraging diverse data sources for predictive analytics and clinical-decision support. The authors state that the ultimate objectives are to enhance the quality of clinical care whilst streamlining its delivery. However, the rapid pace of diabetes AI innovation, coupled with a limited evidence base, presents challenges to achieving safe, integrated and ethically responsible adoption. Mackenzie et al suggest that, to unlock the full potential of AI-enabled diabetes care, stakeholders must collaborate to address issues concerning clinical safety, technological readiness, health equity and user acceptance. The figures from this review are available as a downloadable slideset.
Non‑invasive imaging of sympathetic innervation of the pancreas in individuals with type 2 diabetes – published online 07/11/2023

Achyut Ram Vyakaranam, Maryama M. Mahamed, Per Hellman, Olof Eriksson, Daniel Espes, Gustaf Christoffersson, Anders Sundin
The pancreatic islets of Langerhans are highly innervated, especially from the sympathetic nervous system. Unravelling the complex interplay among neural signals, hormonal regulation and immune responses through quantifiable non-invasive imaging, such as positron emission tomography/computerised tomography (PET/CT) techniques, has been a longstanding pursuit in understanding the pathophysiology of both type 1 and type 2 diabetes. In this issue, Vyakaranam et al (https://doi.org/10.1007/s00125-023-06039-7) explore PET/CT as a potential imaging method for the pancreatic sympathetic nervous system in humans. The PET tracer 11C-hydroxy ephedrine (11C-HED), previously employed to assess cardiac innervation, was used by the authors for diagnosing tumours with a sympathetic origin. Among these individuals examined by 11C-HED-PET in the oncological setting, a lower degree of tracer uptake in the pancreas was found in those with type 2 diabetes, along with regional differences suggesting nerve mass losses. The authors conclude that this study provides a proof of concept for future investigations into pancreatic innervation in both type 1 and type 2 diabetes.
The metabolomic signature of weight loss and remission in the Diabetes Remission Clinical Trial (DiRECT) – published online 25/10/2023

Laura J. Corbin, David A. Hughes, Caroline J. Bull, Emma E. Vincent, Madeleine L. Smith, Alex McConnachie, Claudia‑Martina Messow, Paul Welsh, Roy Taylor, Michael E. J. Lean, Naveed Sattar, Nicholas J. Timpson
The Diabetes Remission Clinical Trial (DiRECT) demonstrated that a structured weight management programme, implemented in a primary care setting, can deliver both meaningful weight loss (24% of participants with more than 15kg weight loss) and type 2 diabetes remission (46% of participants at 12 months). In this issue, Corbin et al use (https://doi.org/10.1007/s00125-023-06019-x) high-throughput metabolomics technologies to explore the wider metabolic consequences of the same intervention. The authors investigated over 1000 metabolites in serum samples collected from 261 participants of the DiRECT trial before and 1 year after beginning the intervention (or control) treatment. Around 14% of all metabolites measured, including many associated with fat metabolism, were found to be altered in the intervention arm. The authors conclude that dietary weight loss reverses many important metabolomic changes previously associated with increased risk of type 2 diabetes, suggesting that excess weight is upstream of many of these metabolic aberrations.
Dyslipidaemia as a target for atherosclerotic cardiovascular disease prevention in children with type 1 diabetes: lessons learned from familial hypercholesterolaemia – published online 30/11/2023

Willemijn E. Corpeleijn, Wouter J. de Waal, Henk S. Schipper, Albert Wiegman
Children with onset of type 1 diabetes under 10 years of age have an 11.4-fold increased risk of premature atherosclerotic cardiovascular disease (ASCVD) compared with matched controls. Part of their ASCVD risk is attributed to dyslipidaemia and, similar to children with familial hypercholesterolaemia (FH), lipid-lowering therapy may lower their ASCVD risk. In this issue, Corpeleijn et al (https://doi.org/10.1007/s00125-023-06041-z) describe how, in a 20 year follow-up study of statins in children with FH, early initiation slowed the progression of carotid intima–media thickness and reduced the risk of ASCVD in adulthood. The authors report that, in their 30 years’ experience of statin use in children with FH they have not observed any serious side effects, such as rhabdomyolysis. Notably, statins have been associated with disease progression in type 2 diabetes, which is likely to be due to an increase in peripheral insulin resistance, a hallmark of type 2 diabetes but less of an issue in type 1 diabetes. The authors conclude that there is a strong rationale for early ASCVD risk management in children with type 1 diabetes, including a potential role for statins. The figure from this review is available as a downloadable slide.
Chronic kidney disease in type 1 diabetes: translation of novel type 2 diabetes therapeutics to individuals with type 1 diabetes – published online 06/10/2023

Vikas S. Sridhar, Christine P. Limonte, Per‑Henrik Groop, Hiddo J. L. Heerspink, Richard E. Pratley, Peter Rossing, Jay S. Skyler, David Z. I. Cherney
Over the past 20 years, the treatment of chronic kidney disease (CKD) in type 1 and type 2 diabetes has focused on glycaemic and blood pressure control, especially, in the latter case, using renin–angiotensin system (RAS) blockers. However, little progress has been made since RAS inhibitor trials demonstrated a slowing of CKD progression, especially in type 1 diabetes. Consequently, individuals with type 1 or type 2 diabetes have a high residual risk of CKD and CVD, and life expectancy for children with type 1 diabetes is reduced, highlighting the urgent need to make progress. However, there is optimism for people with type 2 diabetes. In type 2 diabetes, sodium–glucose cotransporter-2 inhibitors, glucagon-like peptide 1 receptor agonists and non-steroidal mineralocorticoid receptors antagonists have become the standard of care for reducing adverse kidney and cardiovascular outcomes, shifting the focus from a ‘glucose-centric’ to a ‘cardiorenal risk-centric’ approach. In this issue, Sridhar and Limonte et al (https://doi.org/10.1007/s00125-023-06015-1) evaluate the potential translation of these type 2 diabetes therapeutics to individuals with type 1 diabetes, with the lens of preventing the development and progression of CKD. The authors conclude that, considering the mechanistic overlap in the development and progression of CKD in type 1 and type 2 diabetes, there is a strong rationale for developing novel CKD therapies for use in both type 1 and type 2 diabetes and for repurposing existing type 2 diabetes CKD therapies for the treatment of CKD in people with type 1 diabetes. The figure from this review is available as a downloadable slide
Lipotoxicity‑polarised macrophage‑derived exosomes regulate mitochondrial fitness through Miro1‑mediated mitophagy inhibition and contribute to type 2 diabetes development in mice – published online 24/08/2023

Jian‑Ming Li, Xianyu Li, Lawrence W. C. Chan, Ruinian Hu, Tian Zheng, Haojie Li, Sijun Yang
Insulin resistance plays a key role in the development of type 2 diabetes and experimental and clinical studies have shown that insulin resistance can be exacerbated by sustained lipotoxicity-induced mitophagy deficiency. Emerging evidence suggests that exosomes (Exos) from macrophages play an essential role in regulating metabolic homeostasis. In this issue, Li et al (https://doi.org/10.1007/s00125-023-05992-7) show that lipopolysaccharide and palmitic acid-induced macrophages produce M1 Exos, which couple to the mitochondrial transport and fusion machinery and lead to abnormal mitophagy that can promote insulin resistance. The authors report that miR-27-3p is responsible for the effects of lipotoxicity-polarized macrophage-derived M1 Exos both in vitro and in vivo. They show that M1 Exos modulate mitochondrial fitness through upregulation of dynamin-related protein 1 and mitochondrial fission factor and downregulation of mitofusin 2 and optic atrophy protein 1, affecting mitochondrial transport and leading to mitochondrial fission rather than fusion. The authors identify the miR-27-3p–mitochondrial rho GTPase 1 (Miro1) axis as a key insulin-supressing pathway leading to the accumulation of fragmented dysfunctional mitochondria, resulting in a decrease in insulin sensitivity and triggering NOD-like receptor 3-dependent proinflammatory responses. The authors conclude that the miR-27-3p–Miro1 axis could be a new therapeutic target for drug development in type 2 diabetes.
High proinsulin:C‑peptide ratio identifies individuals with stage 2 type 1 diabetes at high risk for progression to clinical diagnosis and responses to teplizumab treatment – published online 04/09/2023

Emily K. Sims, Susan M. Geyer, S. Alice Long, Kevan C. Herold
Heterogeneity exists in type 1 diabetes progression, even within the different stages of the disease. In this issue, Sims et al (https://doi.org/10.1007/s00125-023-06003-5) show that, in individuals with stage 2 type 1 diabetes, an elevation in proinsulin:C-peptide (PI:C) ratio, a biomarker reflecting beta cell stress, is highly predictive of progression to stage 3 type 1 diabetes. They report that teplizumab weakened this relationship between beta cell stress and progression, and participants with a high baseline ratio treated with the drug showed reduced progression rates compared with participants who were treated with placebo. The authors conclude that the PI:C ratio may identify subgroups of individuals who are most likely to benefit from rapid consideration for teplizumab treatment, given their imminent risk of clinical presentation with stage 3 type 1 diabetes.
Clinical impact of an integrated e‑health system for diabetes self‑management support and shared decision making (POWER2DM): a randomised controlled trial – published online 29/09/2023

Merel M. Ruissen, José D. Torres‑Pena, Bas S. Uitbeijerse, Antonio P. Arenas de Larriva, Sasja D. Huisman, Tuncay Namli, Eckhard Salzsieder, Lutz Vogt, Manuela Ploessnig, Bob van der Putte, Armelle Merle, Gustavo Serra, Gustavo Rodríguez, Albert A. de Graaf, Eelco J. P. de Koning, Javier Delgado‑Lista, Jacob K. Sont, POWER2DM Consortium
Diabetes self-management is complex and poses a large burden on individuals. E-health and mobile-health/wireless technologies can be helpful tools to support individuals in their daily diabetes self-management, aiming to reduce this burden. In this issue, Ruissen et al (https://doi.org/10.1007/s00125-023-06006-2) report on the results of a novel e-health support system (POWER2DM) that provides personalised, bidirectional support for both individuals with diabetes and clinicians. The authors describe how POWER2DM integrates biomedical, psychological and behavioural aspects. They show that it is a safe and effective tool that enables both individuals with type 1 diabetes and those with type 2 diabetes to improve glycaemic control, quality of life and diabetes self-management. The authors conclude that e-health and mobile-health support systems that acknowledge the complexity of diabetes care and provide personalised support are helpful tools for improving quality of care and quality of life.
The challenges of identifying and studying type 1 diabetes in adults – published online 20/09/2023

Nicholas J. Thomas, Angus G. Jones
Type 1 diabetes occurs at any age, but diagnosis in adults is difficult as type 2 diabetes predominates. In this issue, Thomas and Jones (https://doi.org/10.1007/s00125-023-06004-4) review the challenges of robustly identifying type 1 diabetes in older adults and outline how these can be addressed. The authors also discuss the potential implications of misclassification for our understanding of type 1 diabetes presenting in adults. The phenotype of type 1 diabetes is commonly reported to be different at older onset ages, overlapping with that of type 2 diabetes. Studies suggest that misclassification of clinically diagnosed type 1 diabetes is increasingly common with older age. The authors state that the inadvertent study of participants with and without autoimmune aetiology diabetes may explain many of the reported changes in the characteristics of those with type 1/autoimmune diabetes with older age. However, when robust disease definitions are used, the phenotype of older-onset type 1 diabetes appears broadly similar to that of type 1 diabetes occurring earlier in life, without differences in presentation, genotype or progression across adult-onset ages. The authors conclude that, in the clinic, biomarker investigation is essential for the diagnosis of adult-onset type 1 diabetes, while, in research, use of high-specificity approaches to define type 1 diabetes in adults is critical to understanding the phenotype of adult-onset autoimmune aetiology diabetes. The figures from this review are available as a downloadable slideset
Quantifying beta cell function in the preclinical stages of type 1 diabetes – published online 15/09/2023

Alfonso Galderisi, Alice L. J. Carr, Mariangela Martino, Peter Taylor, Peter Senior, Colin Dayan
The recent approval by the US Food and Drug Administration of the use of teplizumab in preclinical (stage 2) type 1 diabetes represents a paradigm shift in our therapeutic approach to this disease. Rather than focusing on improvements in insulin replacement, the development of low-risk agents to preserve beta cell function in the preclinical phases of the disease may avert the need for insulin, providing years of burden-free life with near-perfect glucose control. However, to develop new drugs in this space, it is of pivotal importance to be able to accurately measure changes in beta cell function before significant rises in glucose occur, using methods that can be applied in large clinical trial populations. In this issue, Galderisi et al (https://doi.org/10.1007/s00125-023-06011-5) describe the metabolic changes occurring during the preclinical stages of type 1 diabetes and discuss the pros and cons of the available methodologies to quantify beta cell function in these early disease phases. They state that metabolic modelling of the data derived from standard tests, such as the OGTT or mixed meal tolerance test, may provide more accurate estimates of insulin secretion and insulin sensitivity in early-stage type 1 diabetes than C-peptide measurement alone. The authors conclude that such models should be validated in large longitudinal cohorts to confirm their value as effective measures of beta cell function in the early stage of the disease. The figures from this review are available as a downloadable slideset
Glucose‑lowering effects of a synbiotic combination containing Pediococcus acidilactici in C. elegans and mice – published online 16/08/2023

Deyan Yavorov‑Dayliev, Fermín I. Milagro, Josune Ayo, María Oneca, Ignacio Goyache, Miguel López‑Yoldi, Paula Aranaz
In the recent past, the importance of the gut microbiota in the regulation of glucose and insulin homeostasis has been demonstrated. This has led to the emergence of probiotics and synbiotics as alternative therapies to ameliorate metabolic diseases-related disturbances, including those associated with diabetes mellitus, insulin resistance and inflammation. In this issue, Yavorov-Dayliev et al (https://doi.org/10.1007/s00125-023-05981-w) fully characterise the glycaemia-normalising activity of a synbiotic containing Pediococcus acidilactici, oat β-glucans and chromium picolinate in both Caenorhabditis elegans and mice. The authors demonstrate that supplementation with this synbiotic counteracted diabetes-related disturbances in C. elegans following exposure to high glucose and in mice with diet-induced obesity. Specifically, the synbiotic counteracted the effect of the high glucose/diet-induced obesity by modulating the insulin–IGF-1 signalling pathway, and by ameliorating glucose tolerance, excess visceral adiposity, insulin resistance, hepatic steatosis and liver damage. The authors propose that the synbiotic induced these affects by altering the intestinal microbiota, affecting the insulin signalling pathway, activating fatty acid β-oxidation and reducing the low-grade inflammation. In summary, Yavorov‑Dayliev and colleagues suggest that the synbiotic used in their study could provide an alternative strategy for the prevention of type 2 diabetes and its comorbidities.
SERCA2 regulates proinsulin processing and processing enzyme maturation in pancreatic beta cells – published online 04/08/2023

Hitoshi Iida, Tatsuyoshi Kono, Chih‑Chun Lee, Preethi Krishnan, Matthew C. Arvin, Staci A. Weaver, Timothy S. Jarvela, Renato C. S. Branco, Madeline R. McLaughlin, Robert N. Bone, Xin Tong, Peter Arvan, Iris Lindberg, Carmella Evans‑Molina
Impaired processing of proinsulin into mature insulin is a key pathological feature of both type 1 and type 2 diabetes. In this issue, Iida and Kono et al (https://doi.org/10.1007/s00125-023-05979-4) investigate the link between endoplasmic reticulum (ER) Ca2+ levels and proinsulin processing using a mouse model with beta cell-specific sarcoendoplasmic reticulum Ca2+ ATPase-2 (SERCA2) deletion (βS2KO mice). βS2KO mice exhibited age-dependent glucose intolerance and elevated plasma and pancreatic proinsulin levels, whilst, in βS2KO islets, ER Ca2+ levels were reduced and glucose-stimulated Ca2+ synchronicity was impaired. In addition, expression of connexin-36, which is involved in the coordination of Ca2+ oscillations and glucose-stimulated insulin secretion, was reduced in βS2KO islets. Mechanistic studies showed that SERCA2 loss was associated with reduced maturation and activity of proinsulin processing enzymes and resulted in aberrant accumulation of proinsulin in the proximal secretory pathway. Treatment of islets from human donors without diabetes with high glucose and palmitate concentrations partially phenocopied the observations in βS2KO islets. The authors conclude that their findings suggest that chronic ER Ca2+ depletion due to SERCA2 deficiency impairs the spatial regulation of prohormone trafficking and processing within the beta cell.
Potential preventive properties of GLP‑1 receptor agonists against prostate cancer: a nationwide cohort study – published online 03/08/2023

Charlotte Skriver, Søren Friis, Lotte B. Knudsen, Andrei‑Mircea Catarig, Alice J. Clark, Christian Dehlendorff, Lina S. Mørch
Findings from preclinical and epidemiological studies have suggested that glucagon-like peptide-1 receptor agonists (GLP-1RAs) may protect against prostate cancer, but the evidence is inconclusive. In this issue, Skriver et al (https://doi.org/10.1007/s00125-023-05972-x) report findings from a large population-based study employing data from Danish nationwide prescription, cancer, health and administrative registries. Using these data, the authors examined the risk of prostate cancer in a large sample of men aged ≥50 years with diabetes who commenced use of GLP-1RAs or basal insulin during 2007–2019. They show that men treated with GLP-1RAs had a lower incidence of prostate cancer compared with men treated with basal insulin. This association was observed particularly among older men (≥70 years) and men with cardiovascular disease. The authors conclude that their results may indicate that GLP-1RA use could have protective properties against prostate cancer but that further studies are needed to confirm these findings.
Islet autoimmunity in human type 1 diabetes: initiation and progression from the perspective of the beta cell – published online 25/07/2023

Peter J. Thompson, Jasmine Pipella, Guy A. Rutter, Herbert Y. Gaisano, Pere Santamaria
Islet autoimmunity results from a complex dialogue between the immune system and islets, eventually leading to symptomatic type 1 diabetes. In this issue, Thompson and colleagues (https://doi.org/10.1007/s00125-023-05970-z) summarise the various ways in which beta cells influence the onset and progression of islet autoimmunity in type 1 diabetes in humans. Recent work suggests that islet autoimmunity in genetically predisposed individuals results from environmental triggers that may affect beta cells early in life. Beta cells present novel antigens, undergo diverse stress responses and exhibit a functional hierarchy within the islet. Emerging work also points to alpha cells as a potential therapeutic target for arresting islet autoimmunity. Although there are many questions remaining, continued efforts to understand islet autoimmunity through the lens of the beta cell will undoubtedly improve the diagnosis and treatment of type 1 diabetes. The figures from this review are available as a downloadable slideset.
An unwelcome inheritance: childhood obesity after diabetes in pregnancy – published online 13/07/2023

Claire L. Meek
Over 20 million infants per year are born to mothers with diabetes, and are at high risk of childhood obesity, attributed primarily to developmental influences in utero. The early onset of obesity in children with existing environmental and genetic susceptibilities to diabetes should be a major public health concern. In this issue, Claire Meek (https://doi.org/10.1007/s00125-023-05965-w) summarises the current understanding of the pathophysiology of obesity in children after intrauterine exposure to maternal hyperglycaemia. Meek proposes a new hypothesis for the mechanisms underlying childhood obesity in infants born to mothers with diabetes, involving subtle upregulation of de novo lipogenesis pathways and pancreatic beta cell function, which is initiated in utero and persists into childhood. The author also highlights possible opportunities for intervention and concludes that effective intervention will require a new focus on maternal health before, during and after pregnancy to halt the intergenerational cycle of obesity. The figure from this review is available as a downloadable slide.
Hyperglucagonaemia in diabetes: altered amino acid metabolism triggers mTORC1 activation, which drives glucagon production – published online 22/07/2023

Yael Riahi, Aviram Kogot‑Levin, Liat Kadosh, Bella Agranovich, Assaf Malka, Michael Assa, Ron Piran, Dana Avrahami, Benjamin Glaser, Eyal Gottlieb, Fields Jackson III, Erol Cerasi, Ernesto Bernal‑Mizrachi, Aharon Helman, Gil Leibowitz
Diabetes is characterised by hyperglucagonemia as well as insulin deficiency, making it a dual hormone disease; however, the mechanisms involved in alpha cell dysfunction are unclear. In this issue, Riahi et al (https://doi.org/10.1007/s00125-023-05967-8) highlight the nutrient sensor mammalian target of rapamycin complex 1 (mTORC1) as a key player in diabetes-related hyperglucagonemia. They show that mTORC1 activity was increased in alpha cells from type 1 and type 2 diabetes models, and its inhibition by inducible Rptor knockout in alpha cells from a type 1 diabetes model dampened glucagon secretion and ameliorated diabetes. Metabolomics, metabolic flux and gene expression studies revealed that alpha cell exposure to hyperglycaemia enhanced glucose-derived amino acid synthesis and transport, culminating in increased glutamate, branched-chain amino acid and methionine cycle activity, all contributing to stimulation of mTORC1 activation. The authors highlight that prolonged high glucose exposure therefore alters amino acid metabolism, which may drive persistent mTORC1 activation and subsequent excessive glucagon secretion. They conclude that early normalisation of blood glucose levels is crucial to prevent alpha cell dysfunction in diabetes and suggest targeting nutrient(s) metabolism and mTORC1 signalling in alpha cells as an appealing avenue for diabetes treatment.
Strength training is more effective than aerobic exercise for improving glycaemic control and body composition in people with normal‑weight type 2 diabetes: a randomised controlled trial – published online 26/07/2023

Yukari Kobayashi, Jin Long, Shozen Dan, Neil M. Johannsen, Ruth Talamoa, Sonia Raghuram, Sukyung Chung, Kyla Kent, Marina Basina, Cynthia Lamendola, Francois Haddad, Mary B. Leonard, Timothy S. Church, Latha Palaniappan
Previous studies in people with overweight/obesity and type 2 diabetes have shown that a combination of aerobic and resistance training is superior to either type of exercise alone for lowering HbA1c levels. In this issue, Kobayashi et al (https://doi.org/10.1007/s00125-023-05958-9) describe the STRONG-D study, which aimed to determine the impact of different exercise regimens on glycaemic control in people with ‘normal-weight type 2 diabetes’ (BMI <25 kg/m²). The study compared strength training alone, aerobic training alone, and combined strength and aerobic training. In contrast to previous trials in individuals with overweight/obesity, the authors show that strength training alone was more effective at reducing HbA1c levels than aerobic training alone, with combination training showing intermediate effects. The authors highlight that increased lean mass relative to fat mass, observed only in the strength training group, independently predicted lower HbA1c levels. The authors emphasise the significance of strength training for managing type 2 diabetes in normal-weight individuals and highlight the importance of considering body composition in exercise recommendations for this population. They conclude that these findings could contribute to personalised care for different diabetes phenotypes.
SGLT2i and GLP‑1 RA therapy in type 1 diabetes and reno‑vascular outcomes: a real‑world study – published online 28/07/2023

Matthew Anson, Sizheng S. Zhao, Philip Austin, Gema H. Ibarburu, Rayaz A. Malik, Uazman Alam
The beneficial extra-glycaemic effects of SGLT2i and GLP-1 RA on weight, renal protection and major adverse cardiovascular events are well established and make them attractive therapies in type 2 diabetes compared with other more traditional glucose-lowering agents. People with type 1 diabetes share many of the same cardiovascular risk factors as those with type 2 diabetes. Such novel agents are not approved for type 1 diabetes but are still prescribed off-label, with a paucity of robust data underpinning their safety and efficacy in this cohort. In this issue, Anson et al (https://doi.org/10.1007/s00125-023-05975-8) undertake a retrospective analysis of individuals with type 1 diabetes adjunctively treated with either an SGLT2i or a GLP-1 RA, with outcomes analysed 5 years after initiation of therapy. The authors show that individuals treated with an SGLT2i had a reduced risk of developing heart failure and chronic kidney disease and of being hospitalised for any cause compared with those adjunctively treated with a GLP-1 RA, despite an increased risk of diabetic ketoacidosis. They conclude that the findings suggest a net overall benefit of SGLT2i in type 1 diabetes compared with GLP-1 RA therapy and that dedicated long-term randomised trials are warranted to validate these findings.
Incretins: turning the venom into the antidote

In our October 2023 issue, we feature a series of reviews that focus on incretin-based therapies. These drugs were developed following the discovery of a peptide, exendin-4, in the Gila monster’s venom in the 1990s. Being structurally similar to the incretin hormone glucagon-like peptide-1 (GLP-1), exendin-4 was found to mimic the glucose-regulating effects of incretins. The decades following this discovery have seen the generation of several incretin-based therapies and, in this issue of Diabetologia, we are excited to include eight reviews summarising the state-of-the-art knowledge about these agents. Drucker and Holst (https://doi.org/10.1007/s00125-023-05906-7) start by describing the function of GLP-1, namely glucose-dependent potentiation of insulin secretion and glucoregulatory actions, appetite reduction and cardioprotection. Nauck and Müller (https://doi.org/10.1007/s00125-023-05956-x) go on to discuss another incretin hormone: glucose-dependent insulinotropic polypeptide (GIP). GIP was not initially considered an obvious drug candidate; however, a novel drug, tirzepatide, has demonstrated that dual agonism of GLP-1 and GIP receptors produces more substantial reductions in HbA1c and body weight than selective GLP-1 receptor agonists (GLP-1RAs). Tirzepatide is but one example of a novel incretin-based therapy, with this and other advances in incretin pharmacology and drug development being summarised in the review by Tschöp et al (https://doi.org/10.1007/s00125-023-05929-0). These therapies, old and new, not only have therapeutic potential in type 2 diabetes, but also may be beneficial in other types of diabetes. In their review, Mathieu and Ahmadzai (https://doi.org/10.1007/s00125-023-05980-x) discuss the evidence for the beneficial effects of incretin-based therapies in type 1 diabetes, monogenic forms of diabetes and other conditions leading to hyperglycaemia. In terms of diabetic complications, Solini et al (https://doi.org/10.1007/s00125-023-05973-w) delve into the cardiovascular protection offered by incretin-based therapies, while Goldney et al (https://doi.org/10.1007/s00125-023-05988-3) discuss their effects on microvascular complications. Andreasen et al (https://doi.org/10.1007/s00125-023-05966-9) highlight the use of these drugs in the treatment of other metabolic diseases, specifically obesity and non-alcoholic fatty liver disease (NAFLD). Thus, the potential benefits of incretin-based therapies are clearly extensive. In contrast, however, as discussed by Karagiannis et al (https://doi.org/10.1007/s00125-023-05962-z), their uptake is restricted due to socioeconomic factors, such as affordability, accessibility, health literacy and provider bias. To extend their benefits at a societal level, a concerted effort must be made to address these issues. Looking ahead, the future holds great promise for incretin-based therapies to expand the treatment options available for individuals with metabolic disorders, offering new avenues for effective management and improved quality of life. This review set is accompanied by an editorial by Krook and Mulder (https://doi.org/10.1007/s00125-023-05987-4).
Cholesterol crystal formation is a unifying pathogenic mechanism in the development of diabetic retinopathy – published online 14/06/2023

Sandra S. Hammer, Tim F. Dorweiler, Delaney McFarland, Yvonne Adu‑Agyeiwaah, Natalia Mast, Nicole El‑Darzi, Seth D. Fortmann, Sunil Nooti, Devendra K. Agrawal, Irina A. Pikuleva, George S. Abela, Maria B. Grant, Julia V. Busik
With the advancement of spectral-domain optical coherence tomography imaging, hyperreflective crystalline deposits have been identified in retinal pathologies, including diabetic retinopathy. In this issue, Hammer and Dorweiler et al (https://doi.org/10.1007/s00125-023-05949-w) uncover the nature of crystalline deposits in retina from human donors with diabetes as cholesterol crystals. Using cell culture- and animal model-based studies, cholesterol crystals were shown to recapitulate all major pathogenic mechanisms leading to diabetic retinopathy, including inflammation, cell death and breakdown of the blood–retinal barrier. Fibrates, statins and α-cyclodextrin effectively dissolved cholesterol crystals and prevented endothelial pathology. The authors conclude that the formation of cholesterol crystals represents a unifying pathogenic mechanism in the development of diabetic retinopathy and strategies for removal of cholesterol crystals may have therapeutic value in the treatment of diabetic retinopathy.
Low birthweight is associated with a higher incidence of type 2 diabetes over two decades independent of adult BMI and genetic predisposition – published online 12/06/2023

Rasmus Wibaek, Gregers S. Andersen, Allan Linneberg, Torben Hansen, Niels Grarup, Anne Cathrine B. Thuesen, Rasmus T. Jensen, Jonathan C. K. Wells, Kasper A. Pilgaard, Charlotte Brøns, Dorte Vistisen, Allan A. Vaag
Over the past three decades, longitudinal studies have consistently found lower birthweight to be associated with higher risk of type 2 diabetes, but prospective data on diabetes incidence are lacking. In this issue, Wibaek et al (https://doi.org/10.1007/s00125-023-05937-0) used data on objectively measured birthweight from original midwife records dating back to 1939−1971, and in a large sample of middle-aged to older adults examined the influence of birthweight on age- and sex-specific incidence of type 2 diabetes over two decades, from 1999−2020. The authors show that type 2 diabetes incidence rate increased with age, was higher in male participants, and that the absolute rate of increase was markedly higher in individuals born with lower birthweight compared with higher birthweight in a dose−response manner. Altogether, birthweight, genetic susceptibility of type 2 diabetes and adult adiposity (BMI) were found to be strong and independent risk factors for type 2 diabetes. The authors conclude that, within the era of precision medicine, birthweight holds strong potential to be used as a feasible marker to guide clinical care and treatment in type 2 diabetes.
Plasma proteomic signatures of a direct measure of insulin sensitivity in two population cohorts – published 17/06/2023

Daniela Zanetti, Laurel Stell, Stefan Gustafsson, Fahim Abbasi, Philip S. Tsao, Joshua W. Knowles, RISC Investigators, Björn Zethelius, Johan Ärnlöv, Beverley Balkau, Mark Walker, Laura C. Lazzeroni, Lars Lind, John R. Petrie, Themistocles L. Assimes
The euglycaemic−hyperinsulinaemic clamp (EIC) is a reference standard for directly assessing insulin sensitivity but is invasive and time-consuming. In this issue, Zanetti et al (https://doi.org/10.1007/s00125-023-05946-z) assess the incremental value of high-throughput plasma proteomic profiling, using the proximity extension assay, in developing signatures that correlate with the M value derived from the EIC. The authors use two cohorts, the Relationship between Insulin Sensitivity and Cardiovascular disease (RISC) and the Uppsala Longitudinal Study of Adult Men (ULSAM), to show that plasma proteomic signatures of up to 67 proteins substantially improve the cross-sectional estimation of the M value over routinely available clinical variables. A smaller subset of proteins afforded much of this improvement, especially when considering predictive models applied across both cohorts. IGF-binding protein 2 was the single most consistently selected protein across all analyses and models. Zanetti and colleagues state that their approach provides opportunities to improve the identification of individuals at risk of adverse health consequences related to insulin resistance.
Genetics of diabetes‑associated microvascular complications – published online 14/07/2023
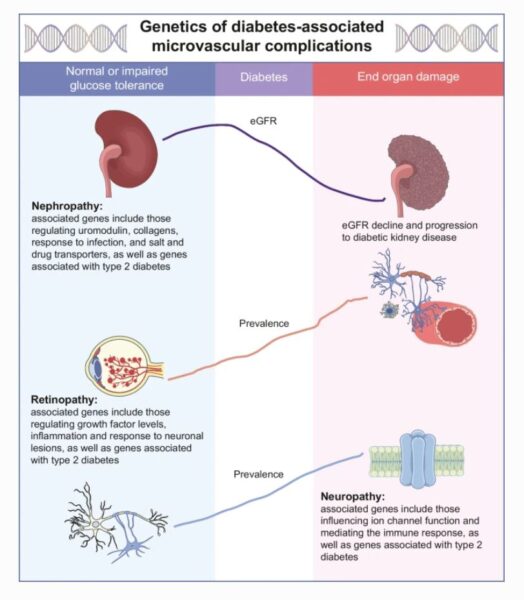
Valeriya Lyssenko, Allan Vaag
The diabetes epidemic has resulted in an epidemic of diabetes-associated complications. Systemic monitoring of individuals with diabetes and new insights into biological mechanisms leading to the progression of complications are necessary to halt this escalation. In this issue, Lyssenko and Vaag (https://doi.org/10.1007/s00125-023-05964-x) summarise state-of-the-art discoveries in the genetic predisposition to kidney, eye and nerve damage in individuals with diabetes. They also provide a critical view on the existing gaps in the current clinical definitions of organ damage that might hinder discovery of genomic factors that trigger or cause associated disease. Knowledge about environmental perinatal exposures may shed light on adaptive changes responsible for the intrauterine programming of metabolic mechanisms that may underlie organ vulnerability. Profiling genetic susceptibility to diabetes-associated metabolic risk factors, including high blood glucose levels, impaired insulin secretion and action, obesity, hypertension, reduced liver function and dysregulated immune system, may aid in pathophysiology-based classification of complications and identification of individuals at high risk for these complications for early prevention in individuals with diabetes. The figure from this review is available as a downloadable slide
Utility of genetic risk scores in type 1 diabetes – published online 13/07/2023

Amber M. Luckett, Michael N. Weedon, Gareth Hawkes, R. David Leslie, Richard A. Oram, Struan F. A. Grant
Advances in genetic research have greatly expanded our understanding of the genetic contribution to type 1 diabetes, facilitating the development of genetic risk scores (GRSs) for type 1 diabetes risk. In this review, Luckett et al (https://doi.org/10.1007/s00125-023-05955-y) summarise the utility of type 1 diabetes GRSs, specifically for disease classification and prediction. They highlight how progression from simplistic models to models that incorporate HLA interactions have allowed us to capture disease risk and discriminate type 1 diabetes from other forms of diabetes. Alongside other factors, such as family history and autoantibody status, GRSs have been integrated into combined risk scores for type 1 diabetes onset prediction. Within newborn population screening, type 1 diabetes GRSs have the potential to identify infants at risk of future presentation of the disease so that they can receive additional clinical care. The authors conclude that the integration of GRSs into healthcare has huge potential for identifying and informing treatment in individuals with type 1 diabetes. The figures from this review are available as a downloadable slideset
Disruption of cortical cell type composition and function underlies diabetes‑associated cognitive decline – published online 23/06/2023

Karis Little, Aditi Singh, Angel Del Marco, María Llorian‑Salvador, Maria Vargas‑Soria, Mireia Turch‑Anguera, Montse Solé, Noëlle Bakker, Sarah Scullion, Joan X. Comella, Ingeborg Klaassen, Rafael Simó, Monica Garcia‑Alloza, Vijay K. Tiwari, Alan W. Stitt, on behalf of the RECOGNISED consortium
People with type-2 diabetes are at higher risk of cognitive decline and dementia; however, the cellular changes that occur in the brain as type 2 diabetes progresses remain poorly understood. In this issue, Little, Singh and Del Marco et al (https://doi.org/10.1007/s00125-023-05935-2) describe using single-cell RNA sequencing to investigate changes to the neurovascular unit (NVU) within the cerebral cortex in a mouse model of type 2 diabetes. The authors identified distinct transcriptional signatures in a number of key neuronal, glial vascular and immune cells, demonstrating that metabolic and inflammatory processes are dysregulated in the cortical glia of diabetic mice. In parallel, they report that neuronal maturation was significantly affected in the type 2 diabetes cortex, with these changes occurring alongside evident cognitive decline and vascular damage. They further demonstrate that post-mortem cortex from individuals with type 2 diabetes showed comparable changes to what was observed in the mouse model. The authors conclude that altered metabolic function, neuroinflammation and changes to neuronal maturation may play an integral role in NVU damage and thus cognitive decline in type 2 diabetes.
Inhibition of the type 1 diabetes candidate gene PTPN2 aggravates TNF-α-induced human beta cell dysfunction and death – published online 29/03/2023

Arturo Roca-Rivada, Sandra Marín-Cañas, Maikel L. Colli, Chiara Vinci, Toshiaki Sawatani, Lorella Marselli, Miriam Cnop, Piero Marchetti, Decio L. Eizirik
TNF-α inhibition delays the progression of type 1 diabetes and circulating TNF-α is associated with aggressive forms of the disease. In this issue, Roca-Rivada et al (https://doi.org/10.1007/s00125-023-05908-5) describe the molecular mechanisms triggered by TNF-α that lead to human beta cell dysfunction and death when the type 1 diabetes candidate gene PTPN2 is silenced. Cells silenced for PTPN2 are more susceptible to the deleterious effect of TNF-α and IFN-α, showing increased beta cell apoptosis. The authors demonstrate that beta cell apoptosis is abolished by the parallel blocking of Bcl-2-like protein 2 (BIM) or c-Jun N-terminal kinase (JNK1), indicating an unexpected common pathway between TNF-α and IFN-α. They further identify JNK1 as a substrate for PTPN2 in beta cells. The authors conclude that people with type 1 diabetes carrying risk-associated PTPN2 polymorphisms may benefit from therapies that inhibit TNF-α.
Umbilical cord‑derived mesenchymal stromal cells preserve endogenous insulin production in type 1 diabetes: a Phase I/II randomised double‑blind placebo‑controlled trial – published online 24/05/2023

Per-Ola Carlsson, Daniel Espes, Sofia Sisay, Lindsay C. Davies, C. I. Edvard Smith and Mathias G. Svahn
Mesenchymal stromal cells (MSCs) have been shown to modulate the immune system and dampen inflammatory and autoimmune responses in numerous diseases. In this issue, Carlsson et al (https://doi.org/10.1007/s00125-023-05934-3) report their findings from a Phase I/II dose escalation and double-blind placebo-controlled clinical trial investigating the Wharton’s jelly MSC drug product, ProTrans, for the treatment of new-onset type 1 diabetes. In the dose escalation safety study, the authors demonstrate that ProTrans can be safely administered intravenously with no serious adverse events. A fixed dose of 200 million MSCs preserved the production of endogenous insulin and reduced exogenous insulin replacement compared with placebo 1 year after treatment. The authors conclude that a single treatment with ProTrans could potentially delay type 1 diabetes disease progression, thereby reducing the associated complications and improving quality of life.
100 years of glucagon and 100 more – published online 27/06/2023

Nicolai J. Wewer Albrechtsen, Jens J. Holst, Alan D. Cherrington, Brian Finan, Lise Lotte Gluud, Danielle Dean, Jonathan E. Campbell, Stephen R. Bloom, Tricia M.-M. Tan, Filip K. Knop, Timo D. Müller
More than 100 years ago, scientists were on the path to discovering a central novel metabolic regulator, now known as glucagon. Although the role of glucagon in diabetes has been studied intensively, its place in physiology and pathophysiology is still debated. In this issue, Wewer Albrechtsen et al (https://doi.org/10.1007/s00125-023-05947-y) capture the fundamentals of glucagon biology and its role in metabolic diseases. Key questions on how glucagon secretion is controlled, not only by glucose but also by amino acids and lipids, are addressed. In addition, the authors discuss how a new concept, termed ‘glucagon resistance’, may explain the diabetogenic hyperglucagonaemia observed in metabolic diseases. The authors propose that future glucagon research may help to uncover the molecular backbone of inter-organ dysfunction in individuals with diabetes and liver disease. They conclude that, as well as treating hypoglycaemia, glucagon-based therapies may also provide benefits for weight loss and the treatment of fatty liver disease. The figures from this review are available as a downloadable slideset
Patient-reported outcomes for people with diabetes: what and how to measure? A narrative review – published online 24/05/2023

Caroline B. Terwee, Petra J. M. Elders, Marieke T. Blom, Joline W. Beulens, Olaf Rolandsson, Alize A. Rogge, Matthias Rose, Nicola Harman, Paula R. Williamson, Frans Pouwer, Lidwine B. Mokkink, Femke Rutters
Patient-reported outcomes (PROs) are important for shared decision making and standardisation of outcomes in research. However, in the field of diabetes, the use of PROs and associated patient-reported outcome measures (PROMs) is heterogeneous. A core outcome set for clinical trials and an International Consortium for Health Outcomes Measurement (ICHOM) standard set for clinical practice have been developed, but they, as well as other initiatives, recommend different PROs and PROMs. Standardisation of relevant outcomes and outcome measures is therefore needed. In this issue, Terwee et al (https://doi.org/10.1007/s00125-023-05926-3) provide recommendations on the selection of relevant PROs and PROMs for use in clinical practice and research in people with diabetes. The figure from this review is available as a downloadable slide
GLP-1R agonists demonstrate potential to treat Wolfram syndrome in human preclinical models – published online 30/03/2023

Vyron Gorgogietas, Bahareh Rajaei, Chae Heeyoung, Bruno J. Santacreu, Sandra Marín-Cañas, Paraskevi Salpea, Toshiaki Sawatani, Anyishai Musuaya, María N. Arroyo, Cristina Moreno-Castro, Khadija Benabdallah, Celine Demarez, Sanna Toivonen, Cristina Cosentino, Nathalie Pachera, Maria Lytrivi, Ying Cai, Lode Carnel, Cris Brown, Fumihiko Urano, Piero Marchetti, Patrick Gilon, Decio L. Eizirik, Miriam Cnop, Mariana Igoillo-Esteve
Wolfram syndrome is an autosomal recessive disorder caused by mutations in the WFS1 gene. Individuals affected by Wolfram syndrome develop diabetes mellitus, optic nerve atrophy, hearing loss and other neurological problems. There are currently no treatments to prevent or delay the disease. However, glucagon-like peptide 1 receptor (GLP-1R) agonists have been shown to preserve glucose tolerance and reduce neuroinflammation and vision loss in Wfs1-deficient mice and rats. In this issue, Gorgogietas et al (https://doi.org/10.1007/s00125-023-05905-8) report that GLP-1R agonists also improve the function and survival of WFS1-deficient human pancreatic beta cells and neurons. The authors conclude that these data provide a strong preclinical basis to test GLP-1R agonists in individuals with Wolfram syndrome in clinical trials.
Engineered allele substitution at PPARGC1A rs8192678 alters human white adipocyte differentiation, lipogenesis, and PGC‑1α content and turnover – published online 12/05/2023

Mi Huang, Melina Claussnitzer, Alham Saadat, Daniel E. Coral, Sebastian Kalamajski, Paul W. Franks
Genetic association studies have correlated hundreds of loci with metabolic disorders, but the functional basis of these loci is rarely explored. A well-known common genetic polymorphism in PPARGC1A (rs8192678, C/T, Gly482Ser) has been reproducibly associated with obesity and type 2 diabetes in various ancestries, highlighting the need to examine its allele-specific effects and pinpoint its clinical relevance. In this issue, Mi Huang et al (https://doi.org/10.1007/s00125-023-05915-6) report the use of a state-of-the-art CRISPR/Cas9 technique to generate isogenic adipose cell lines with different rs8192678 genotypes. They show that the rs8192678 T allele causally enhances adipogenic differentiation and mitochondrial function in an allele dosage-dependent manner. They also demonstrate that the T allele is associated with higher levels of the PPARGC1A-encoded peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) protein and of the adipogenesis master regulator peroxisome proliferator-activated receptor γ (PPARγ). These findings provide experimental insights into adipocyte-specific mechanisms underlying epidemiological correlations between rs8192678 and metabolic disorders. The authors conclude that this may prove useful for the development of genotype-based precision medicine for obesity.
Pen-administered low-dose dasiglucagon vs usual care for prevention and treatment of non-severe hypoglycaemia in people with type 1 diabetes during free-living conditions: a Phase II, randomised, open-label, two-period crossover trial – published online 11/04/2023

Christian Laugesen, Ajenthen G. Ranjan, Signe Schmidt, Kirsten Nørgaard
Consumption of excess carbohydrate to manage hypoglycaemia can lead to rebound hyperglycaemia and promote weight gain. Previous inpatient studies have demonstrated that s.c. low-dose glucagon can be used to effectively treat non-severe hypoglycaemia in people with type 1 diabetes, but studies in outpatient settings are limited. In this issue, Laugesen et al (https://doi.org/10.1007/s00125-023-05909-4) report the findings of a randomised clinical study comparing the efficacy of pen-administered low-dose dasiglucagon with that of usual care for the prevention and treatment of non-severe hypoglycaemia during free-living conditions. The authors show that use of low-dose dasiglucagon was safe, fast and efficacious while significantly reducing the total daily carbohydrate intake and yielding high treatment satisfaction. The authors conclude that their results add to existing evidence suggesting that pen-administered low-dose dasiglucagon has the potential to become a new and non-caloric method of managing non-severe hypoglycaemia for individuals with type 1 diabetes.
Effects of postprandial exercise on blood glucose levels in adults with type 1 diabetes: a review – published online 04/04/2023

Simon Helleputte, Jane E. Yardley, Sam N. Scott, Jan Stautemas, Laura Jansseune, Joke Marlier, Tine De Backer, Bruno Lapauw, Patrick Calders
In people with type 1 diabetes, blood glucose management around exercise can be very challenging, especially if exercise is performed shortly (within 2 h) after a meal (i.e. in the early postprandial period) when circulating insulin levels are high. In this issue, Helleputte, Yardley et al (https://doi.org/10.1007/s00125-023-05910-x) summarise the available data on the glycaemic effects of postprandial exercise in people with type 1 diabetes. They state that an enhanced understanding of the effects of postprandial exercise on blood glucose can help to improve blood glucose management around physical activity in this population. The studies included in this review show that prandial status is an important determinant of the blood glucose response to exercise in type 1 diabetes, as several modalities of postprandial exercise (walking and continuous and interval exercise) resulted in a decline in blood glucose concentration. The authors suggest that mealtime insulin reductions are needed to provide safe glycaemic profiles during exercise and, thereby, avoid exercise-induced hypoglycaemia. However, they highlight that issues remain concerning hyperglycaemia around exercise and late-onset post-exercise hypoglycaemia. The authors conclude that more research is needed into strategies to improve blood glucose management around postprandial exercise in people with type 1 diabetes. The figure from this review is available as a downloadable slide
The countdown to type 1 diabetes: when, how and why does the clock start? – published online 26/05/2023

Anette-Gabriele Ziegler
The first years of life are characterised by an enormous number of new exposures that challenge and shape our immune system and metabolism. It is during this period that genetically susceptible children have the highest risk of developing autoimmunity against beta cells. In this issue, Anette-Gabriele Ziegler (https://doi.org/10.1007/s00125-023-05927-2) reviews the interplay between the environment, immunity and metabolism in an attempt to explain why this fertile period for the development of islet autoimmunity in early childhood is critical for the development of type 1 diabetes. The figures from this review are available as a downloadable slideset
The extent and magnitude of islet T cell infiltration as powerful tools to define the progression to type 1 diabetes – published online 08/03/2023

Paola S. Apaolaza, Diana Balcacean, Jose Zapardiel-Gonzalo, Teresa Rodriguez-Calvo
Immune infiltration in the islets of Langerhans is a hallmark of type 1 diabetes. However, there is a lack of understanding of infiltration dynamics in terms of magnitude (i.e. how many immune cells are present) and extent (i.e. in how many islets). In this issue, Apaolaza et al (https://doi.org/10.1007/s00125-023-05888-6) characterise T cell infiltration by investigating islets with moderate and high levels of infiltration in the human pancreas. The authors show that about a third of islets have moderate infiltration in double autoantibody-positive and type 1 diabetic donors, while islets with high infiltration are less abundant. Likewise, these donors have high islet and exocrine T cell density, suggesting that, as disease progresses, T cell infiltration extends throughout the pancreas, reaching both the islets and exocrine compartment. The authors conclude by presenting new analytical tools with the aim of modelling how T cells infiltrate the pancreas, and estimating pancreatic infiltration in living individuals.
Altered blood gene expression in the obesity-related type 2 diabetes cluster may be causally involved in lipid metabolism: a Mendelian randomisation study – published online 24/02/2023

Juliette A. de Klerk, Joline W. J. Beulens, Hailiang Mei, Roel Bijkerk, Anton Jan van Zonneveld, Robert W. Koivula, Petra J. M. Elders, Leen M. ’t Hart, Roderick C. Slieker
Individuals with diabetes are a heterogenous group and therefore further stratification is key. It has previously been shown that individuals with type 2 diabetes can be organised into five clusters based on five clinical variables: age, HbA1c, BMI, C-peptide level and HDL-cholesterol level. While clusters differ in terms of clinical outcomes, the differences at the molecular level are largely unclear. In this issue, de Klerk et al (https://doi.org/10.1007/s00125-023-05886-8) compare whole blood gene expression profiles between the different clusters of people with diabetes. The authors show that relatively young people with a high BMI have an altered blood transcriptome profile compared with the other clusters. Using Mendelian randomisation, they demonstrate that the differentially expressed mRNAs may have a causal effect on multiple traits, including anthropometric characteristics and lipid metabolism. The authors conclude that the clusters may help to further stratify people with diabetes, highlighting the different underlying pathophysiologies and providing a more holistic view of type 2 diabetes.
Diabetes and climate change: current evidence and implications for people with diabetes, clinicians and policy stakeholders – published online 25/03/2023

Jacqueline M. Ratter-Rieck, Michael Roden, Christian Herder
Climate change and associated environmental risk factors, such as extreme weather events, air pollution and altered host–pathogen interactions, are a threat to human health. In this issue, Ratter-Rieck et al (https://doi.org/10.1007/s00125-023-05901-y) summarise recent evidence on how climate change may affect people with diabetes. The authors discuss how impaired responses to heat stress, diabetes-associated comorbidities and specific clinical characteristics make people with diabetes particularly vulnerable to climate-change-associated health risks, leading to increased risks of morbidity and mortality, for instance during heatwaves or after extreme weather events. They highlight that studies identifying additional predisposing factors and high-risk groups will support the development of targeted interventions. The authors conclude that implementation of climate change adaptation and mitigation strategies at governmental, clinical and individual levels will help to limit the detrimental health effects of climate change on people with diabetes. The figures from this review are available as a downloadable slideset
Sex differences in type 2 diabetes – published online 10/03/2023

Alexandra Kautzky-Willer, Michael Leutner, Jürgen Harreiter
Our understanding of the sex and gender differences in type 2 diabetes has increased over the past decade. In this issue, Kautzky-Willer et al (https://doi.org/10.1007/s00125-023-05891-x) summarise recent advances in our knowledge of sex-specific clinical features of type 2 diabetes, and the differences between women and men in risk, diagnosis, management and outcomes of type 2 diabetes. The authors discuss how, overall, men have a slightly higher prevalence of type 2 diabetes diagnosis at a younger age while being less obese. Psychosocial stress is a more prominent diabetes risk factor for women than men, and women have a greater cardiometabolic risk factor burden than men at the time of diagnosis. Pregnancy complications, especially gestational diabetes, and early menopause increase the risk of type 2 diabetes in women, while low testosterone levels are associated with a higher risk in men. The authors conclude that more aggressive risk management needs to be implemented for both men and women who are at increased risk of type 2 diabetes, as well as for those with established diabetes. The figures from this review are available as a downloadable slideset
Evidence-based European recommendations for the dietary management of diabetes – published online 17/04/2023

The Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD)
Diabetes management relies on effective evidence-based guidance to inform individuals and support them to improve their health. In this issue, the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD) (https://doi.org/10.1007/s00125-023-05894-8) provides an update to its 2004 recommendations on the dietary management of diabetes. These guidelines are primarily for health professionals and consider nutrients, foods, food groups, patterns and some of the broader lifestyle aspects of what we eat. The guidelines draw on a substantial body of published work, much of which was conducted to inform this update, and include recommendations on prevention, management and remission. The guidelines provide effective evidence-based advice to inform the conversation between health professionals and their patients, and empower individuals to manage their health.
Chaperonin counteracts diet-induced non-alcoholic fatty liver disease by aiding sirtuin 3 in the control of fatty acid oxidation – published online 24/01/2023

Shao-Wen Weng, Jian-Ching Wu, Feng-Chih Shen, Yen-Hsiang Chang, Yu-Jih Su, Wei-Shiung Lian, Ming-Hong Tai, Chia-Hao Su, Jiin-Haur Chuang, Tsu-Kung Lin, Chia-Wei Liou, Tian-Huei Chu, Ying-Hsien Kao, Feng-Sheng Wang, Pei-Wen Wang
Heat shock protein 60 (HSP60) is a mitochondrial chaperonin that plays an important role in escorting unfolded proteins. Mice deficient in HSP60 develop mitochondrial dysfunction and insulin resistance; however, the biological role of this chaperonin in nutrient metabolism and the development of non-alcoholic fatty liver disease (NAFLD) remains unclear. In this issue, Wang et al (https://doi.org/10.1007/s00125-023-05869-9) report that HSP60 deficiency was correlated with severe steatosis in human NAFLD biopsies. In contrast, transgenic mice overexpressing Hsp60 (Hsp60-Tg) developed less body fat, showed amelioration of dyslipidaemia, hepatic steatosis and M1/M2 macrophage dysregulation and exhibited lower levels of insulin resistance than wild-type mice when fed a high-fat diet. The respiratory quotient profile indicated that fat in Hsp60-Tg mice may be metabolised to meet energy demands. The authors demonstrate that, mechanistically, HSP60 promoted fatty acid oxidation by preserving sirtuin 3 (SIRT3)/AMP-activated protein kinase (AMPK)/peroxisome proliferator-activated receptor α (PPARα) signalling. The authors conclude that gain of mitochondrial HSP60 function may be a promising avenue for the development of therapeutic interventions for NAFLD and type 2 diabetes.
Presence of immunogenic alternatively spliced insulin gene product in human pancreatic delta cells – published online 08/03/2023

René van Tienhoven, Maria J. L. Kracht, Arno R. van der Slik, Sofia Thomaidou, Anouk H. G. Wolters, Ben N. G. Giepmans, Juan Pablo Romero Riojas, Michael S. Nelson, Françoise Carlotti, Eelco J. P. de Koning, Rob C. Hoeben, Arnaud Zaldumbide, Bart O. Roep
In type 1 diabetes, insulin-producing beta cells in the pancreatic islets of Langerhans contribute to their own demise in various ways. Under stress, beta cells can generate so-called neoantigens that result from misreads from insulin mRNA (e.g. insulin defective ribosomal product [INS-DRiP]) and which strongly provoke the immune system. In this issue, van Tienhoven et al (https://doi.org/10.1007/s00125-023-05882-y) report on the surprising finding that an antibody generated against these new beta cell stress proteins selectively stains delta cells. The authors show that the target of this antibody is another insulin gene product, resulting from alternative splicing of insulin mRNA (referred to as INS-splice), that partly overlaps with INS-DRiP. Islet delta cells express this insulin gene product, INS-splice, which contains important targets of diabetes-causing T cells. The authors highlight that this finding may point to some delta cells being potential targets of autoimmunity. The authors conclude that insulin splicing may also play a role in islet development and senescence.
Separate and combined effects of semaglutide and empagliflozin on kidney oxygenation and perfusion in people with type 2 diabetes: a randomised trial – published online 06/02/2023

Søren Gullaksen, Liv Vernstrøm, Steffen S. Sørensen, Steffen Ringgaard, Christoffer Laustsen, Kristian L. Funck, Per L. Poulsen, Esben Laugesen
Diabetes mellitus is the leading cause of chronic kidney disease. Kidney hypoxia has been suggested as a unifying pathophysiological pathway in the development of chronic kidney disease in diabetes. Renoprotective effects have been documented for the sodium−glucose cotransporter 2 inhibitor empagliflozin whereas positive effects for the glucagon-like peptide-1 receptor agonist semaglutide await confirmation in dedicated kidney outcome trials. The underlying mechanisms of action of the two drugs are unclear, but it has been suggested that they may improve kidney oxygenation. In this issue, Gullaksen et al (https://doi.org/10.1007/s00125-023-05876-w) report that treatment with empagliflozin for 32 weeks, in contrast to previous assumptions, decreases kidney medullary oxygenation in people with type 2 diabetes. Semaglutide did not affect kidney medullary oxygenation nor was there any additional effect on oxygenation with combination therapy. The authors suggest that empagliflozin-induced medullary hypoxia may stimulate erythropoietin production, leading to kidney protection. They conclude that these findings improve our understanding of the differential kidney protective effects of empagliflozin and semaglutide.
Causal factors underlying diabetes risk informed by Mendelian randomisation analysis: evidence, opportunities and challenges – published online 14/02/2023

Shuai Yuan, Jordi Merino, Susanna C. Larsson
Exploration of causal factors underlying diabetes is of great importance not only for the development of more effective prevention strategies but also to provide insight into the molecular processes underlying disease risk. Mendelian randomisation is an epidemiological method that can strengthen causal inference based on the use of genetic variation. In this issue, Yuan et al (https://doi.org/10.1007/s00125-023-05879-7) summarise the evidence on potential causal risk factors for diabetes by integrating published Mendelian randomisation studies on type 1 and 2 diabetes, and reflect on future perspectives of Mendelian randomisation studies on diabetes. The authors highlight that, despite the influence of genetics on type 1 diabetes, few Mendelian randomisation studies have been conducted to identify causal exposures or molecular processes leading to increased disease risk. For type 2 diabetes, Mendelian randomisation analyses support causal associations of somatic, mental and lifestyle factors with development of the disease. The authors discuss how studies on circulating protein biomarkers, metabolites and gut microbiota provide valuable data to better understand disease pathophysiology and explore potential therapeutic targets. They conclude that more Mendelian randomisation studies in multi-ancestry cohorts are needed to examine the role of different types of physical activity, dietary components, metabolites, protein biomarkers and gut microbiome in diabetes development The figure from this review is available as a downloadable slide.
Embracing complexity: making sense of diet, nutrition, obesity and type 2 diabetes – published online 14/02/2023
Nita G. Forouhi
Diet and nutrition are critical for the prevention and mitigation of type 2 diabetes. However, the research evidence and its implementation have been challenging, partly because of the plethora of information and apparently conflicting dietary strategies. In this issue, Nita Forouhi (https://doi.org/10.1007/s00125-023-05873-z) reviews the evidence on the role of dietary components in the prevention and management of type 2 diabetes and obesity. The review cuts through the complexity of, and challenges of measuring, diet and investigates the impact of diet using robust study designs. The author concludes that it is unhelpful to play off one nutrient against another, such as favouring a low-fat or a low-carbohydrate diet. Instead, the review suggests the importance of nutrient type or quality, nutrient food sources in food-based guidelines and the relevance of overall dietary patterns, as well as taking into account dietary adherence and longer term effects. Important areas of consensus on effective dietary strategies are highlighted and future directions are considered. The figure from this review is available as a downloadable slide.
Loss of RREB1 in pancreatic beta cells reduces cellular insulin content and affects endocrine cell gene expression – published online 12/01/2023

Katia K. Mattis, Nicole A. J. Krentz, Christoph Metzendorf, Fernando Abaitua, Aliya F. Spigelman, Han Sun, Jennifer M. Ikle, Swaraj Thaman, Antje K. Rottner, Austin Bautista, Eugenia Mazzaferro, Marta Perez-Alcantara, Jocelyn E. Manning Fox, Jason M. Torres, Agata Wesolowska-Andersen, Grace Z. Yu, Anubha Mahajan, Anders Larsson, Patrick E. MacDonald, Benjamin Davies, Marcel den Hoed, Anna L. Gloyn
Genome-wide association studies have identified multiple independent signals at the RREB1 locus associated with type 2 diabetes. However, how altered expression or function of the transcription factor Ras-responsive element binding protein 1 (RREB1) influences diabetes risk was previously unknown. In this issue, Mattis and Krentz et al (https://doi.org/10.1007/s00125-022-05856-6) describe how a combination of zebrafish and human cellular models was used to identify disease-causing mechanisms at the RREB1 locus. The authors show how RREB1 loss-of-function reduced insulin gene expression and insulin content in zebrafish as well as in human beta cell models. Transcriptomic analysis identified RREB1 as a regulator of several genes involved in beta cell development and function, including the RFX family of transcription factors. Consistent with these findings, the authors show how isolated islets from human carriers of RREB1 diabetes-risk alleles exhibited altered glucose-stimulated insulin secretion. The authors conclude that the genetic association of RREB1 with type 2 diabetes is mediated, in part, by a transcriptional role for RREB1 in normal beta cell development and function.
Inequalities in cancer mortality trends in people with type 2 diabetes: 20 year population-based study in England – published online 24/01/2023

Suping Ling, Francesco Zaccardi, Eyad Issa, Melanie J Davies, Kamlesh Khunti, Karen Brown
Owing to improvements in cardiovascular disease prevention and treatment in the past few decades, mortality rates in people with type 2 diabetes have declined substantially in some high-income countries. Given the increased incidence and mortality for some cancers associated with diabetes, it is unclear whether cancer has overtaken cardiovascular disease as the key cause of death in this population and whether inequalities exist in cancer mortality trends. In this issue, Ling et al (https://doi.org/10.1007/s00125-022-05854-8) report that, in contrast to declining all-cause mortality rates in people with type 2 diabetes at all ages between 1998 and 2018, there were decreasing trends in all-cancer mortality rates at younger ages but increasing trends at older ages (75+). In addition, they show that there were persistent inequalities in cancer mortality rates by gender and socioeconomic status and widening disparities by smoking status. The authors conclude that these findings highlight that cancer deserves a similar level of attention as other diabetes-related complications, such as cardiovascular disease, and that public health policies are needed to address persistent and widening inequalities.
Projecting the incidence and costs of major cardiovascular and kidney complications of type 2 diabetes with widespread SGLT2i and GLP 1 RA use: a cost-effectiveness analysis – published online 21/11/2022

Jedidiah I. Morton, Clara Marquina, Jonathan E. Shaw, Danny Liew, Kevan R. Polkinghorne, Zanfina Ademi and Dianna J. Magliano
Sodium–glucose co-transporter 2 inhibitors (SGLT2is) and glucagon-like peptide‑1 receptor agonists (GLP-1 Ras) reduce the incidence of cardiovascular and kidney disease in addition to their effects on blood glucose. However, it is unclear if they are cost-effective on the basis of their cardiovascular and kidney benefits alone, which may be why many payers/governments have HbA1c-based restrictions on their use. In this issue, Morton et al (https://doi.org/10.1007/s00125-022-05832-0) report that, based solely on their cardiovascular benefits at current prices, SGLT2is are cost-effective for anyone with type 2 diabetes from the Australian healthcare perspective, while GLP-1 RAs are unlikely to be cost-effective, even in a population with pre-existing cardiovascular disease. The authors conclude that these findings suggest that existing HbA1c-based restrictions on SGLT2i use may not be justified from a health economic perspective.
Missing the forest-plot for the trees – published online 14/01/2023

Deirdre K. Tobias
Systematic reviews and meta-analyses are respectable research tools when used correctly. In this issue, Deirdre Tobias (https://doi.org/10.1007/s00125-022-05862-8) describes, however, that the quality of systematic reviews today is highly variable, despite standard operating procedures and best practices, warranting serious concerns about over-reliance on their findings without paying careful attention to potential bias. She discusses how this has undoubtedly led to some arguments against their use and value to the scientific community (see the counter-debate by Enzo Bonora in this issue). However, she goes on to highlight that dismissing this critical and growing evidence base altogether would be a disservice to rigorous scientific progress. She concludes that researchers should instead be encouraged to improve their proficiency in reading, conducting and interpreting systematic review research so that these reviews better serve their intended role as efficient synthesisers of accumulating evidence and gatekeepers of redundant original research.
The ‘scientist’, the ‘analyst’ and the ‘novelist’: science or metrics? – published online 22/12/2022

Enzo Bonora
Progress in medicine relies on scientific literature because journals are the main peer-reviewed medium of discoveries, achievements, concepts and ideas, inspiring further research as well as establishing best clinical practices. In this issue, Enzo Bonora (https://doi.org/10.1007/s00125-022-05808-0) illustrates the huge increase in diabetes-related literature over the last few decades but highlights what he believes is an excess of ‘nothing-to-add’ papers and, in particular, redundant meta-analyses and repetitive narrative reviews. He also emphasises the enthusiasm that meta-analyses and reviews receive from some journals and some scientists, anxious to improve their own metrics. He concludes that the scientific relevance of papers and the scientific achievements of investigators and journals are more important for medical progress than their respective metrics. See the counter-debate by Deirdre Tobias in this issue.
CCR2-positive monocytes contribute to the pathogenesis of early diabetic retinopathy in mice – published online 26/01/2023

Aicha Saadane, Alexander A. Veenstra, Martin S. Minns, Jie Tang, Yunpeng Du, Fatima Abubakr Elghazali, Emma M. Lessieur, Eric Pearlman, Timothy S. Kern
Inflammation has been implicated in the pathogenesis of the early stages of diabetic retinopathy but the molecular mechanisms are unclear. In this issue, Saadane et al (https://doi.org/ 10.1007/s00125-022-05860-w) that deletion of CC chemokine receptor 2 (CCR2)-positive cells (largely monocytes) in a mouse model of diabetes or generation of chimeric mice lacking Ccr2 only from myeloid cells significantly inhibited the diabetes-induced increase in retinal capillary degeneration. The authors highlight how these results in monocytes reflect previous studies that have shown that neutrophils have direct cytotoxic effects on retinal endothelial cells, thus providing at least one mechanism by which leucocytes contribute to diabetes-induced vascular damage in diabetes. They conclude that abnormalities in multiple cell types in the innate immune system contribute to the development of the early stages of diabetic retinopathy, providing potential therapeutic targets for inhibiting retinopathy.
High-throughput genetic clustering of type 2 diabetes loci reveals heterogeneous mechanistic pathways of metabolic disease – published online 20/12/2022

Hyunkyung Kim, Kenneth E. Westerman, Kirk Smith, Joshua Chiou, Joanne B. Cole, Timothy Majarian, Marcin von Grotthuss, Soo Heon Kwak, Jaegil Kim, Josep M. Mercader, Jose C. Florez, Kyle Gaulton, Alisa K. Manning, Miriam S. Udler
Genome-wide association studies (GWAS) have identified hundreds of loci associated with type 2 diabetes; however, clinical translation of findings has been challenging. In this issue, Kim et al (https://doi.org/10.1007/s00125-022-05848-6) describe a high-throughput pipeline using GWAS summary statistics to perform physiologically informed clustering of 323 independent type 2 diabetes loci and identified ten genetic clusters. These clusters represent subsets of diabetes risk variants that are most similar to each other based on their associations with disease-related traits. The ten clusters included both previously captured and novel clusters, and displayed tissue-specific enrichment of epigenomic marks. Cluster-based polygenic scores were associated with distinct clinical outcomes. The authors also demonstrated application of the pipeline to two other metabolic diseases. They conclude that their high-throughput clustering approach can produce robust findings and identify potential genetic subtypes of disease.
Exercise as a non-pharmacological intervention to protect pancreatic beta cells in individuals with type 1 and type 2 diabetes – published online 19/11/2022

Alexandra Coomans de Brachène, Corentin Scoubeau, Anyïshai E. Musuaya, Jose Maria Costa-Junior, Angela Castela, Julie Carpentier, Vitalie Faoro, Malgorzata Klass, Miriam Cnop, Decio L. Eizirik
Exercise training is known to reduce diabetes risk. In this issue, Coomans de Brachène et al (https://doi.org/10.1007/s00125-022-05837-9) report that serum obtained from 82 individuals after 8–12 weeks of exercise training protects human pancreatic beta cells against apoptosis induced by the endoplasmic reticulum stressor thapsigargin, compared with serum obtained from the same individuals before training. The protective effect was observed regardless of the type of exercise training, or sex, age, BMI, ancestry or diabetes status (type 1, type 2 or non-diabetic) of the individuals. The study points to a role for muscle-released clusterin in this protective effect, and other exerkines may also be involved. The authors highlight the unexpected potential to preserve beta cell health by exercise training and suggest that exercise could be tested as a non-pharmacological approach to preserve beta cell mass in the early stages of diabetes.
Cardiovascular outcomes in type 1 and type 2 diabetes – published online14/01/2023

Annika Rosengren, Pigi Dikaiou
A typical adult with type 1 diabetes is different from adults with type 2 diabetes, with respect to mean age, duration of diabetes and body size. While individuals with type 2 diabetes are generally older and have higher levels of other cardiometabolic risk factors, those with type 1 typically have longer exposure to high plasma glucose levels. In this issue, Rosengren and Dikaiou (https://doi.org/10.1007/s00125-022-05857-5) discuss why both types of diabetes face increased risk of cardiovascular disease. The authors highlight that in each type of diabetes, absolute risk and excess risk, compared with the general population, vary depending on the control of other factors. However, few studies have formally compared the two types, and those studies that have done so have reached different conclusions. Accordingly, there is as yet no consensus on which type of diabetes fares worse with respect to cardiovascular disease. The authors conclude that future studies in large, unselected populations are needed, taking other risk factors into account. The figures from this review are available as a downloadable slideset.
Importance of beta cell mass for glycaemic control in people with type 1 diabetes – published online 17/11/2022

T. J. P. Jansen, M. Brom, M. Boss, M. Buitinga, C. J. Tack, L. A. van Meijel, B. E. de Galan, M. Gotthardt
The importance of residual beta cells for glucose control in people with type 1 diabetes is not yet fully understood and measurement of human beta cell mass is challenging. In this issue, Jansen et al (https://doi.org/10.1007/s00125-022-05830-2) report the novel use of an imaging method, exendin PET, to measure beta cell mass in a non-invasive manner. The authors demonstrate that beta cell mass was markedly increased in people with type 1 diabetes and relatively stable glycaemic control than in people with type 1 diabetes and high glucose variability. They suggest that residual beta cells may therefore play an important role in glycaemic stability in people with type 1 diabetes. The authors highlight that their finding indicates the need for effective therapies aimed at preserving viable beta cells and therapies that can restore or improve beta cell functionality. They conclude that exendin PET may have a role to play in the detection of these residual beta cells and could aid in the selection of the most suitable treatments.
Outdoor light at night in relation to glucose homoeostasis and diabetes in Chinese adults: a national and cross-sectional study of 98,658 participants from 162 study sites – published online 14/11/2022

Ruizhi Zheng, Zhuojun Xin, Mian Li, Tiange Wang, Min Xu, Jieli Lu, Meng Dai, Di Zhang, Yuhong Chen, Shuangyuan Wang, Hong Lin, Weiqing Wang, Guang Ning, Yufang Bi, Zhiyun Zhao, Yu Xu
Artificial lighting has increased significantly during recent decades and detrimental effects of artificial light at night (LAN) exposure on metabolic health have been reported. However, it is unclear whether exposure to outdoor artificial LAN is associated with glucose homoeostasis and diabetes. In this issue, Zheng and Xin et al (https://doi.org/10.1007/s00125-022-05819-x) used data from a national survey of the general population in China and report that chronic exposure to outdoor LAN was positively associated with blood glucose levels, insulin resistance and diabetes prevalence and inversely associated with beta cell function. The authors highlight that, considering the acceleration in urbanisation and growing number of domestic migrants arriving in large cities, the number of people with light pollution-related diabetes is projected to increase. They conclude that effective prevention and intervention policies should be developed to protect people from the adverse health effects of light pollution at night.
Global trends in the incidence of hospital admissions for diabetes-related foot disease and amputations: a review of national rates in the 21st century – published online 13/12/2022

Peter A. Lazzarini, Susanna M. Cramb, Jonathan Golledge, Jedidiah I. Morton, Dianna J. Magliano, Jaap J. Van Netten
Diabetic foot disease is a leading cause of hospitalisation and amputation. In this issue, Lazzarini et al (https://doi.org/10.1007/s00125-022-05845-9) review published data to determine the latest trends in global hospitalisation and amputation rates for diabetic foot disease. The authors provide evidence that global trends in hospitalisation rates for major amputations are largely decreasing but trends in minor amputations and diabetic foot disease are inconsistent. Further, they highlight that hospitalisation rates for diabetic foot disease without amputation are substantially higher than rates for diabetic foot disease with amputation and higher than rates for most other major diabetes complications. However, they suggest the need for caution in the ‘global’ interpretation of these findings because of the high heterogeneity of published data and limited data from low- and middle-income countries. The authors conclude that global reporting standards are needed to better interpret, monitor and address the large global burden of hospitalisation and amputation caused by diabetic foot disease.
Diabetes and the COVID-19 pandemic – published online 23/11/2022

Kamlesh Khunti, Jonathan Valabhji, Shivani Misra
People living with diabetes are at greater risk of hospitalisation and death following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. In addition, the coronavirus disease-2019 (COVID-19) pandemic has disrupted healthcare delivery for people living with diabetes. In this issue, Khunti et al (https://doi.org/10.1007/s00125-022-05833-z) summarise the evidence on the acute impact of COVID-19 on people with diabetes, including data on the occurrence of new-onset diabetes and diabetic ketoacidosis, and the wider impact of the pandemic on healthcare services. The authors conclude by presenting recommendations for prioritising patients with diabetes during the pandemic recovery phase. The figures from this review are available as a downloadable slideset
Targeted proteomics identifies potential biomarkers of dysglycaemia, beta cell function and insulin sensitivity in Black African men and women – published online 17/09/2022

Amy E. Mendham, Lisa K. Micklesfield, Fredrik Karpe, Andre Pascal Kengne, Tinashe Chikowore, Clement N. Kufe, Maphoko Masemola, Nigel J. Crowther, Shane A. Norris, Tommy Olsson, Sölve Elmståhl, Tove Fall, Lars Lind, Julia H. Goedecke
The pathogenesis of type 2 diabetes appears to differ between Black Africans and Europeans, but the diagnostic criteria are the same. In this issue, Mendham et al (https://doi.org/10.1007/s00125-022-05788-1) highlight biomarkers that are both similar and different between Black African and European cohorts who have different lifestyles and sociodemographic profiles. The authors identified 73 proteins associated with impaired glucose metabolism and/or type 2 diabetes in a cohort of middle-aged Black South African men and women, of which 34 proteins were validated in a European cohort. Among the validated proteins, 11 were associated with components of type 2 diabetes pathophysiology such as fasting insulin resistance, peripheral insulin sensitivity and beta cell function. The African-specific biomarkers identified require validation in independent African cohorts to not only identify unique risk markers, but also to increase our understanding of the pathophysiology of type 2 diabetes in African populations.
Loneliness increases the risk of type 2 diabetes: a 20 year follow-up – results from the HUNT study – published online 28/09/2022
Roger E. Henriksen, Roy M. Nilsen, Ragnhild B. Strandberg
A growing body of research has pointed to an association between psychological stress and an individual’s risk of developing type 2 diabetes. In this issue, Henriksen et al (https://doi.org/10.1007/s00125-022-05791-6) report from their 20 year follow-up study showing that participants who recorded a high level of loneliness were twice as likely to develop type 2 diabetes than those who did not feel lonely. Although their study did not examine the exact mechanisms involved, the researchers suggest that loneliness may activate the body’s physiological stress response and that this response may play a central role in the development of type 2 diabetes through different pathways. The authors recommend healthcare providers to be open to dialogue about an individual’s concerns during clinical consultations, including loneliness and social relationships.
Secular trend for increasing birthweight in offspring of pregnant women with type 1 diabetes: is improved placentation the reason? – published online 26/10/2022

Gernot Desoye, Lene Ringholm, Peter Damm, Elisabeth R. Mathiesen, Mireille N. M. van Poppel
In pregnant women with type 1 diabetes, whenever metabolism is disturbed, the fetus is at risk of growing too large. This may lead to complications during delivery and also has long term consequences for the child’s health, including childhood obesity. As such, normalising metabolism during pregnancy would be expected to reduce fetal overgrowth. Paradoxically, however, in the past few decades, improved healthcare and blood glucose control in women with type 1 diabetes, especially in the periconception period, have had the opposite effect, i.e. an increase in birthweight. In this review, Desoye et al (https://doi.org/10.1007/s00125-022-05820-4) discuss that improved blood glucose control may lead to better placentation in women with type 1 diabetes, which exposes the fetus to changes in metabolism more than in earlier decades. The resulting fetal overgrowth may then result in excess fat, independent of birthweight. The authors highlight that this calls for even stricter glucose control during the whole pregnancy period and suggest taking advantage of new diabetes technology. They conclude that evaluation of diabetes management should focus on neonatal fat, rather than birthweight. The figures from this review are available as a downloadable slideset.
Ketones: the double-edged sword of SGLT2 inhibitors? – published online 16/10/2022

Beatrice C. Lupsa, Richard G. Kibbey, Silvio E. Inzucchi
Sodium−glucose cotransporter 2 (SGLT2) inhibitors are a relatively recent class of glucose-lowering medication, originally approved for treating individuals with type 2 diabetes, that have been found to have cardiovascular and renal benefits, independent of their glucose-lowering effect. The mechanisms underlying these salutary effects remain unclear. In this issue, Lupsa et al (https://doi.org/10.1007/s00125-022-05815-1) provide a comprehensive review of the advantages of SGLT2 inhibitors on cardiac and kidney outcomes and the potential role of elevated plasma ketone levels in mediating these benefits. The impact of SGLT2 inhibitors on ketone physiology is reviewed and the risk of ‘euglycaemic’ diabetic ketoacidosis is additionally explored. The topic of ketogenesis and SGLT2 inhibitors is of both scientific and clinical significance.
Automated insulin delivery: benefits, challenges, and recommendations. A Consensus Report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association – published online 06/10/2022

Jennifer L. Sherr, Lutz Heinemann, G. Alexander Fleming, Richard M. Bergenstal, Daniela Bruttomesso, Hélène Hanaire, Reinhard W. Holl, John R. Petrie, Anne L. Peters, Mark Evans
Both clinical studies and real-world experience show that insulin pumps that link to glucose sensors to allow automated insulin delivery (AID) carry great benefits, both in terms of glucose metrics (with lower average blood glucose and increased time in target range) and user experience. However, as with all powerful and effective tools, potential pitfalls exist. In this issue, Sherr et al (https://doi.org/10.1007/s00125-022-05744-z), on behalf of the joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and American Diabetes Association, describe some of these challenges and limitations. Several recommendations are listed for regulators, device companies, diabetes societies, research funders, health care professionals and users of technology. In particular, the authors highlight that AID is not a cure for diabetes, and clinicians need to understand how individual systems work and ensure that people with diabetes using AID are aware of the limitations of current systems.
Sex-specific effects of maternal metformin intervention during glucose-intolerant obese pregnancy on body composition and metabolic health in aged mouse offspring – published online 16/09/2022

Josca M. Schoonejans, Heather L. Blackmore, Thomas J. Ashmore, Lucas C. Pantaleão, Luciana Pellegrini Pisani, Laura Dearden, John A. Tadross, Catherine E. Aiken, Denise S. Fernandez-Twinn, Susan E. Ozanne
Metformin is used to treat gestational diabetes in many countries. Although beneficial for the mother and safe for the neonate, the long-term effects on exposed offspring remain unknown. In this issue, Schoonejans et al (https://doi.org/10.1007/s00125-022-05789-0) report that maternal metformin treatment in a mouse model of obese glucose-intolerant pregnancy leads to sex- and age-specific increases in metabolic risk factors in exposed offspring. The authors propose that fetal metformin exposure can adversely influence adipose tissue development, leading to a postnatal phenotype characterised by adiposity, adipose tissue inflammation and ectopic lipid deposition in tissues such as the liver. The authors conclude that the findings highlight the complexity of balancing short-term beneficial maternal or fetal effects and long-term offspring consequences of placenta-crossing therapies such as metformin, and stress the importance of following up offspring of both sexes throughout life.
Live enteroviruses, but not other viruses, detected in human pancreas at the onset of type 1 diabetes in the DiViD study – published online 12/08/2022

Lars Krogvold, Angelo Genoni, Anna Puggioni, Daniela Campani, Sarah J. Richardson, Christine S. Flaxman, Bjørn Edwin, Trond Buanes, Knut Dahl-Jørgensen, Antonio Toniolo
The team behind the DiViD study previously reported the presence of enterovirus (EV) genome and proteins in pancreatic sections from six live newly diagnosed patients with type 1 diabetes. In this issue, Krogvold et al (https://doi.org/10.1007/s00125-022-05779-2) confirm the presence of EVs and also demonstrate that no other common human viruses are present in the pancreases of the six DiViD cases. The authors demonstrate that the EV strains detected represent live infectious viruses capable of establishing a persistent pancreatic infection. As previously shown in persistent EV infection of the heart, persistent EV infection of the pancreas could lead to progressive tissue-specific dysfunction. The authors conclude that the early phase of type 1 diabetes is associated with low-grade EV infection. They go on to suggest that the findings strengthen the need for studies exploring the potential benefits of enteroviral vaccines for individuals at high risk of developing type 1 diabetes and antiviral treatment for individuals in the early phase of type 1 diabetes.
Normal and disordered gastric emptying in diabetes: recent insights into (patho)physiology, management and impact on glycaemic control – published online 04/10/2022

Ryan J. Jalleh, Karen L. Jones, Christopher K. Rayner, Chinmay S. Marathe, Tongzhi Wu, Michael Horowitz
Gastric emptying is central to the pathogenesis and rational management of type 1 and type 2 diabetes. In this issue, Jalleh et al (https://doi.org/10.1007/s00125-022-05796-1) review the effects of diabetes and its treatment on gastric emptying. The authors discuss how gastric emptying is delayed (i.e. gastroparesis) in 30–50% of people with longstanding, complicated type 1 or type 2 diabetes. In contrast, in well-controlled type 2 diabetes, gastric emptying is often abnormally rapid. Upper gastrointestinal symptoms, which should be assessed routinely using a validated instrument, occur frequently, but the relationship of symptoms with disordered gastric emptying is not simply ‘cause-and-effect’. The longstanding therapeutic approach of making the stomach ‘pump’ better has, predictably, proven unsuccessful. The authors go on to outline how gastric emptying, even when normal, is a key determinant of postprandial blood glucose levels. In insulin-treated diabetes, disordered gastric emptying may predispose an individual to hypoglycaemia/increased blood glucose variability. Both short- and long-acting glucagon-like peptide-1 receptor agonists slow gastric emptying to diminish postprandial glycaemic excursions. The authors conclude that measurement of gastric emptying, using a precise technique, should be incorporated in clinical trials of novel therapies that lower postprandial glucose. The figures from this review are available as a downloadable slideset.
Pathophysiology of type 2 diabetes in sub-Saharan Africans – published online 27/09/2022
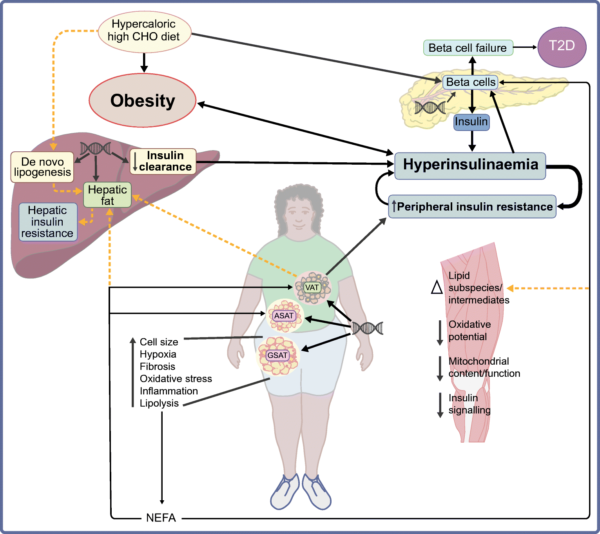
Julia H. Goedecke, Amy E. Mendham
Sub-Saharan Africa (SSA) is the region that is projected to have the greatest rate of increase in type 2 diabetes (129% by 2045). In this issue, Goedecke and Mendham (https://doi.org/10.1007/s00125-022-05795-2) summarise known mechanisms relating to the pathophysiology of type 2 diabetes in sub-Saharan Africans and highlight factors that may influence the pathogenesis of type 2 diabetes in SSA, such as social determinants, infectious diseases and genetic and epigenetic influences. They propose that, among Black Africans from SSA, hyperinsulinaemia due to a combination of both increased insulin secretion and reduced hepatic insulin clearance is the primary defect, which promotes obesity and insulin resistance, exacerbating the hyperinsulinaemia and eventually leading to beta cell failure and type 2 diabetes. The authors conclude that future research on dietary interventions that reduce hyperinsulinaemia and obesity is warranted to gain insights into the mechanistic underpinnings of type 2 diabetes in this population. The figures from this review are available as a downloadable slideset.
Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) – published online 24/09/2022
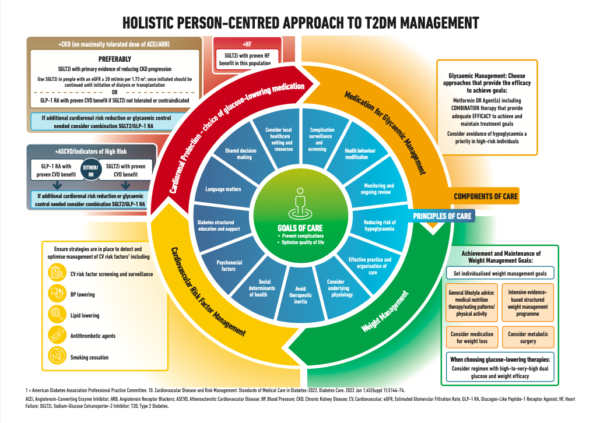
Melanie J. Davies, Vanita R. Aroda, Billy S. Collins, Robert A. Gabbay, Jennifer Green, Nisa M. Maruthur, Sylvia E. Rosas, Stefano Del Prato, Chantal Mathieu, Geltrude Mingrone, Peter Rossing, Tsvetalina Tankova, Apostolos Tsapas, John B. Buse
In 2022, the ADA/EASD updated their consensus report on the management of hyperglycaemia in type 2 diabetes. In this issue, Davies et al (https://doi.org/10.1007/s00125-022-05787-2) present important changes to the 2019 consensus, reflecting new evidence generated in the last 3 years, such as that from cardiovascular and renal outcome trials of sodium–glucose cotransporter-1 inhibitors and glucagon-like peptide-1 receptor agonists, including assessment of subgroups. Recommendations for cardiorenal protection in those with diabetes and at high risk of cardiorenal disease are also presented. The authors focus on holistic management, weight management and consideration of physical behaviours, including sleep, over a 24 h period. The authors also outline that social determinants of health must be addressed if we are to aspire to improve health outcomes. The consensus concludes with a ‘Call to Action’ stating that we must establish and refine quality improvement efforts in diabetes care at local level to equitably implement evidence-based interventions for the benefit of all people living with type 2 diabetes.
Glucose-mediated insulin secretion is improved in FHL2-deficient mice and elevated FHL2 expression in humans is associated with type 2 diabetes – published online 08/07/2022

Jayron J. Habibe, Maria P. Clemente-Olivo, Torsten P. M. Scheithauer, Elena Rampanelli, Hilde Herrema, Mariska Vos, Arnout Mieremet, Max Nieuwdorp, Daniel H. van Raalte, Etto C. Eringa, Carlie J. M. de Vries
FHL2, the gene encoding the four and a half LIM domains 2 (FHL2) protein, contains DNA methylation marks, which forensic studies have consistently found to be correlated with the age of an individual. Hypermethylation of these CpG loci causes an increase in FHL2 expression. In this issue, Habibe and Clemente-Olivo et al (https://doi.org/10.1007/s00125-022-05750-1) show that individuals with type 2 diabetes also express higher FHL2 levels in their pancreatic islets compared with healthy individuals. Furthermore, the authors demonstrate that, compared with their wild-type littermates, Fhl2-deficient mice clear glucose faster, whereas insulin sensitivity is similar for both strains of mice. Isolated pancreatic islets from mice that are deficient for Fhl2 show increased glucose-induced insulin secretion, which the authors suggest may be explained, at least partially, by enhanced expression of the glucose-transporter GLUT2. In line with this, FHL2 gain of function is detrimental to insulin secretion of cultured beta cells due to a reduced uptake of glucose and enhanced levels of reactive oxygen species. The authors conclude that inhibition of FHL2 in human transplant islets may improve transplant function in vivo.
Three weeks of time-restricted eating improves glucose homeostasis in adults with type 2 diabetes but does not improve insulin sensitivity: a randomised crossover trial – published online 25/07/2022

Charlotte Andriessen, Ciarán E. Fealy, Anna Veelen, Sten M. M. van Beek, Kay H. M. Roumans, Niels J. Connell, Julian Mevenkamp, Esther Moonen-Kornips, Bas Havekes, Vera B. Schrauwen-Hinderling, Joris Hoeks, Patrick Schrauwen
Time-restricted eating (TRE) is a form of intermittent fasting whereby food intake is limited to a pre-defined time window during the day. Previous studies in healthy, overweight/obese adults showed that 6–8 h TRE regimes were successful in improving metabolic health. In this issue, Andriessen et al (https://doi.org/10.1007/s00125-022-05752-z) investigated the effect of a more accessible 10 h TRE intervention in adults with type 2 diabetes. Three weeks of TRE resulted in lower fasting glucose and 24 h glucose levels, as well as more time spent in the normal glucose range as compared with spreading habitual food intake over at least 14 h per day. The study did not find changes in insulin sensitivity, hepatic glycogen or substrate oxidation. The authors conclude that these findings highlight the therapeutic potential of TRE in adults with type 2 diabetes. They recommend that more studies are conducted to investigate the underlying mechanisms and long-term effects of TRE.
Islet amyloid polypeptide aggregation exerts cytotoxic and proinflammatory effects on the islet vasculature in mice – published online 25/07/2022

Joseph J. Castillo, Alfred C. Aplin, Daryl J. Hackney, Meghan F. Hogan, Nathalie Esser, Andrew T. Templin, Rehana Akter, Steven E. Kahn, Daniel P. Raleigh, Sakeneh Zraika, Rebecca L. Hull
Aggregation of islet amyloid polypeptide (IAPP) is a pathologic feature of several forms of diabetes, including type 2 diabetes. Aggregated IAPP accumulates in the islet extracellular matrix between beta cells and the islet vasculature and is well known to be cytotoxic to islet beta cells. However, whether IAPP aggregation is also detrimental to the islet vasculature, an important modulator of beta cell function/survival, has not previously been examined. In this issue, Castillo et al (https://doi.org/10.1007/s00125-022-05756-9) use cell- and animal models to show that IAPP elicits a cytotoxic and pro-inflammatory response from cultured islet microvascular endothelial cells. In pancreases from transgenic mice, the authors found that aggregated IAPP (amyloid deposits) exerts specific, localised effects to increase capillary diameter and increase the number of neuron-glial antigen 2 (NG2)-positive islet pericytal structures. The authors conclude that, together, these findings demonstrate that the islet vasculature is a target of the cytotoxic and proinflammatory effects of IAPP, which is likely to contribute to beta cell failure in diabetes.
Intestinal lipid absorption and transport in type 2 diabetes – published online 30/07/2022

Bruno Vergès
The intestine plays an important role in the dyslipidaemia observed in type 2 diabetes and particularly in postprandial hyperlipidaemia, which is known to promote atherosclerosis and increase the incidence of cardiovascular disease. In this issue, Bruno Vergès (https://doi.org/10.1007/s00125-022-05765-8) reviews disorders of intestinal lipid metabolism in type 2 diabetes, which include increased chylomicron production by enterocytes and delayed catabolism of chylomicrons and chylomicron remnants. He outlines how overproduction of chylomicrons is secondary to increased expression of microsomal triglyceride transfer proteins, higher stability and availability of apolipoprotein B-48 and increased de novo lipogenesis. He goes on to discuss how reduced activity of lipoprotein lipase is a major factor responsible for reduced catabolism of chylomicrons in type 2 diabetes. Interestingly, some glucose-lowering treatments significantly influence intestinal lipid metabolism, particularly glucagon-like peptide-1 agonists. Vergès concludes that a better understanding of intestinal lipid metabolism should help to define interesting therapeutic targets for improving postprandial lipid metabolism in type 2 diabetes. The figures from this review are available as a downloadable slideset.
Registry-based randomised clinical trials: a remedy for evidence-based diabetes care? – published online 29/07/2022

Jan W. Eriksson, Björn Eliasson, Louise Bennet, Johan Sundström
Over recent years, registry-based randomised clinical trials (RRCT) have been used in some clinical conditions, for example within cardiology and orthopaedic surgery. In both type 1 and type 2 diabetes, there are several examples of observational studies based on registries or established cohorts that evaluate treatment effects; however, to date, no RRCTs have been performed. In this issue, Eriksson et al (https://doi.org/10.1007/s00125-022-05762-x) review how pragmatic large-scale clinical trials could be applied in the diabetes area. The authors propose that both academic and industry sponsors should consider this highly cost-effective and robust design for future large-scale diabetes trials. Following allocation to randomised treatment, the participants’ outcome data are collected from established healthcare registries or potentially from other well-defined cohort databases. The authors outline the pros and cons of RRCTs compared with traditional RCTs. The first RRCT in diabetes is briefly described, namely the ongoing SMARTEST trial, which is evaluating the effects of monotherapy with a sodium–glucose cotransporter 2 (SGLT2) inhibitor vs metformin to prevent macro- and microvascular events and premature death in patients with early-stage type 2 diabetes. The authors conclude that RRCTs in diabetes could enable the rapid recruitment of large cohorts with broad coverage of both geographical and disease subgroups and provide robust endpoint data at very low cost. The figures from this review are available as a downloadable slideset.
Calcium-dependent transcriptional changes in human pancreatic islet cells reveal functional diversity in islet cell subtypes – published online 26/05/2022
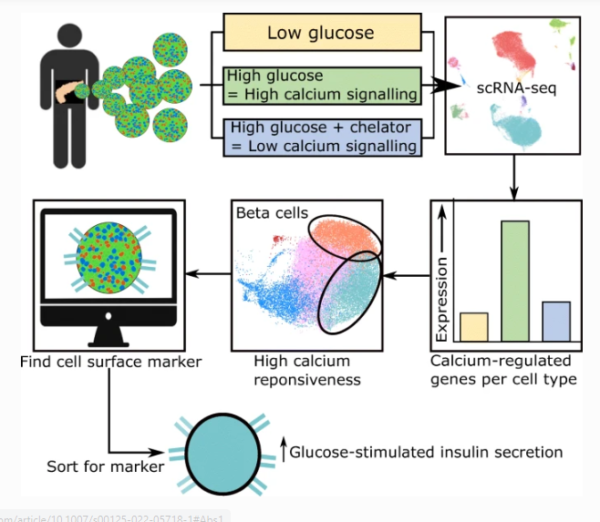
Ji Soo Yoon, Shugo Sasaki, Jane Velghe, Michelle Y. Y. Lee, Helena Winata, Cuilan Nian, Francis C. Lynn
Intracellular calcium is an important secondary messenger that can rapidly couple islet cell electrical activity to gene expression changes. However, the identities of calcium-regulated genes in human islets remain largely unknown. In this issue, Yoon et al (https://doi.org/10.1007/s00125-022-05718-1) profile calcium-regulated genes in human islets by comparing the single-cell transcriptomes of islet cells in the presence or absence of extracellular calcium influx. The authors show that alpha, beta, delta and polyhormonal cell types express calcium-regulated genes that are specific to each cell type. The authors also demonstrate that PCDH7 mRNA is present in beta cells that express the highest number of calcium-regulated genes and that cell surface PCDH7 protein can be used to purify beta cells with enhanced glucose-stimulated insulin secretion. The authors conclude that calcium-regulated transcriptional changes can be used to retrospectively identify different human islet cell subtypes or functional states.
Genome-wide meta-analysis and omics integration identifies novel genes associated with diabetic kidney disease – published online 28/06/2022

Niina Sandholm, Joanne B. Cole, Viji Nair, Xin Sheng, Hongbo Liu, Emma Ahlqvist, Natalie van Zuydam, Emma H. Dahlström, Damian Fermin, Laura J. Smyth, Rany M. Salem, Carol Forsblom, Erkka Valo, Valma Harjutsalo, Eoin P. Brennan, Gareth J. McKay, Darrell Andrews, Ross Doyle, Helen C. Looker, Robert G. Nelson, Colin Palmer, Amy Jayne McKnight, Catherine Godson, Alexander P. Maxwell, Leif Groop, Mark I. McCarthy, Matthias Kretzler, Katalin Susztak, Joel N. Hirschhorn, Jose C. Florez
Diabetic kidney disease is the leading cause of kidney disease. In this issue, Sandholm, Cole et al (https://doi.org/10.1007/s00125-022-05735-0) analysed genetic data from nearly 27,000 individuals with diabetes. These were combined with multiple omics datasets including gene expression, chromatin accessibility and DNA methylation as well as careful morphological characterisation of kidney tissue from nephrectomies and biopsies to identify novel genetic factors and genes that contribute to the risk of diabetic kidney disease. The authors report that several genes—TENM2, DCLK1, AKIRIN2, SNX30 and LSM14A in particular—contribute to the biological processes that lead to diabetic kidney disease and suggest that these genes could be putative therapeutic targets. They also provide evidence that genetic factors for chronic kidney disease in the general population are correlated with those for diabetic kidney disease in type 2 diabetes, but less in type 1 diabetes. The authors also report that the data further confirms the role of obesity in the pathogenesis of diabetic kidney disease.
Obesity in late adolescence and incident type 1 diabetes in young adulthood – published online 05/06/2022

Inbar Zucker, Yair Zloof, Aya Bardugo, Avishai M. Tsur, Miri Lutski, Yaron Cohen, Tali Cukierman-Yaffe, Noga Minsky, Estela Derazne, Dorit Tzur, Cheli Melzer Cohen, Orit Pinhas-Hamiel, Gabriel Chodick, Itamar Raz, Arnon Afek, Hertzel C. Gerstein, Amir Tirosh, Gilad Twig
Excessive weight at birth or in early childhood is linked to an increased risk for type 1 diabetes later in childhood. However, among adolescents who are overweight or with obesity, the future risk for incident type 1 diabetes is less clear. In this issue, Zucker et al (https://doi.org/10.1007/s00125-022-05722-5) report that higher adolescent BMI was related in a severity-dependent manner to an increased risk for type 1 diabetes in young adulthood in a nationwide cohort of 1.4 million Israeli adolescents. The authors suggest that adolescent obesity may double the risk for incident type 1 diabetes even in the absence of other comorbidities, possibly through various cellular pathophysiological processes. The authors conclude that excessive adolescent weight is a potentially modifiable risk factor for incident type 1 diabetes.
Depression, diabetes, comorbid depression and diabetes and risk of all-cause and cause-specific mortality: a prospective cohort study – published online 27/05/2022
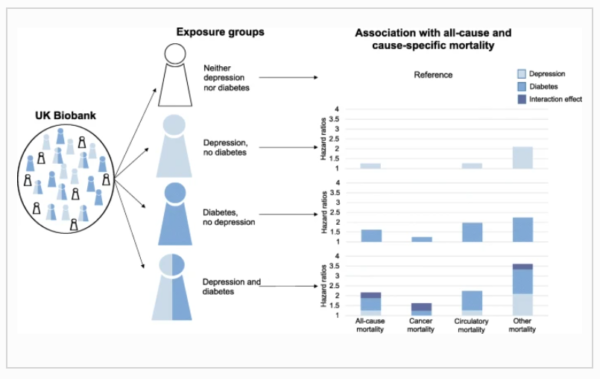
Regina Prigge, Sarah H. Wild, Caroline A. Jackson
Despite the substantial burden of both depression and diabetes, and the potential impact on the prognosis of patients affected by both disorders, limited knowledge exists about the individual and joint effects of depression and diabetes on cause-specific mortality. In this issue, Prigge et al (https://doi.org/10.1007/s00125-022-05723-4) report that individuals with either or both depression and diabetes were generally at higher risk of all-cause mortality and mortality due to cancer, circulatory disease and causes other than circulatory disease or cancer than people with neither condition. The authors show that the association between depression and diabetes was additive for circulatory disease mortality, with synergistic effects observed for cancer mortality and mortality due to causes other than circulatory disease and cancer beyond those expected from their individual effects (i.e. supra-additive effects). The authors conclude that cost-effective interventions for primary and secondary prevention of the individual and joint effects of depression and diabetes are needed.
Diabetic retinopathy screening in the emerging era of artificial intelligence – published online 31/05/2022

Jakob Grauslund
Using screening for the early detection of sight-threatening diabetic retinopathy is a pivotal step towards the reduction of visual loss in diabetes. In this issue, Jakob Grauslund (https://doi.org/10.1007/s00125-022-05727-0) presents the current state-of-the-art in diabetic retinopathy screening and outlines the start of the journey towards the adoption of new technologies and initiatives. These include handheld mobile devices, ocular telehealth programmes and automated image analysis using artificial intelligence. The author focuses on the clinical rationale and scientific evidence for deep learning, which has become the state-of-the-art in computer-based classification and segmentation in medical imaging. The author discusses how first regulatory approvals have been obtained for algorithms trained to detect sight-threatening diabetic retinopathy. He concludes that full-scale implementation in local and national screening programmes can be expected in the upcoming years, once ongoing challenges have been addressed in the transition from in silico experiments to clinical care. The figures from this review are available as a downloadable slideset.
IgM-associated gut bacteria in obesity and type 2 diabetes in C57BL/6 mice and humans – published online 19/05/2022
James A. Pearson, Heyuan Ding, Changyun Hu, Jian Peng, Brittany Galuppo, F. Susan Wong, Sonia Caprio, Nicola Santoro, Li Wen
B cells secrete different immunoglobulins, which can target bacteria. IgA-deficiency promotes obesity through changes to the composition of gut bacteria; however, IgA deficiency often increases IgM. In this issue, Pearson, Ding and Hu et al (https://doi.org/10.1007/s00125-022-05711-8) used activation-induced cytidine deaminase (AID)-deficient mice (which produce only IgM) fed on a high fat diet to investigate the role of IgM in obesity. They show that increased IgM promoted weight gain and impaired glucose- and insulin tolerance by altering the composition of the intestinal bacteria in the mice. Administration of intravenous IgG, but not IgA, abolished the obesogenic profile of AID-deficient mice. The authors also showed that in obese children with impaired glucose tolerance and type 2 diabetes, IgM-bound stool bacteria are increased compared with normoglycaemic children without type 2 diabetes. Additionally, gut bacteria derived from either AID-deficient obese mice or obese children with impaired glucose tolerance and type 2 diabetes induced similar metabolic changes in germ-free mice. The authors conclude that these findings indicate that IgM may be important in the development of obesity and type 2 diabetes.
Insulin-degrading enzyme ablation in mouse pancreatic alpha cells triggers cell proliferation, hyperplasia and glucagon secretion dysregulation – published online 02/06/2022

Beatriz Merino, Elena Casanueva-Álvarez, Iván Quesada, Carlos M. González-Casimiro, Cristina M. Fernández-Díaz, Tamara Postigo-Casado, Malcolm A. Leissring, Klaus H. Kaestner, Germán Perdomo, Irene Cózar-Castellano
Hyperglucagonaemia is a hallmark of type 2 diabetes, although its underlying mechanisms are poorly elucidated. Insulin-degrading enzyme (IDE) is a protease of insulin and glucagon which is highly expressed in human and mouse pancreatic alpha cells, although its expression levels are decreased in the pancreatic islet cells of individuals with type 2 diabetes. In this issue, Merino et al (https://doi.org/10.1007/s00125-022-05729-y) report that deletion of IDE in adult mouse alpha cells leads to increased proliferation, hyperplasia and constitutively elevated glucagon secretion, with lack of inhibition by insulin or high-glucose levels, leading to hyperglucagonaemia. Furthermore, they demonstrate that IDE deficiency triggers cytoskeletal perturbations, including increased α-synuclein aggregation and decreased tubulin levels, in parallel to impaired ciliogenesis in alpha cells. The authors conclude that these findings highlight novel molecular mechanisms of glucagon secretion regulation in pancreatic alpha cells, which may represent a future therapeutic target to treat hyperglucagonaemia in type 2 diabetes.
Mediators of the association between educational attainment and type 2 diabetes mellitus: a two-step multivariable Mendelian randomisation study – published online 28/04/2022

Jia Zhang, Zekai Chen, Katri Pärna, Sander K. R. van Zon, Harold Snieder, Chris H. L. Thio
Higher educational attainment protects against type 2 diabetes, but the underlying mechanisms are uncertain. In this issue, Zhang et al (https://doi.org/10.1007/s00125-022-05705-6) report the results of a Mendelian randomisation study, in which they used genetic instruments to minimise bias due to confounding. Using a multivariable extension of this method, they estimated mediation between educational attainment and type 2 diabetes by the modifiable risk factors BMI, sedentary behaviour, smoking and blood pressure. They estimate that up to 84% of the protective effect of higher educational attainment is mediated by lower levels of these risk factors. The two largest mediating factors were BMI and sedentary behaviour, each individually mediating 50% of the protective effect, with partially overlapping effects. The authors conclude that these findings might inform future trials and preventive policies to reduce the burden of type 2 diabetes due to educational inequalities.
Health impact of seven herpesviruses on (pre)diabetes incidence and HbA1c: results from the KORA cohort – published online 11/05/2022

Tim Woelfle, Birgit Linkohr, Tim Waterboer, Barbara Thorand, Jochen Seissler, Marc Chadeau-Hyam, Annette Peters
During the COVID-19 pandemic, the link between viral infections and non-communicable diseases has once again become apparent. In this issue, Woelfle et al (https://doi.org/10.1007/s00125-022-05704-7) investigate the link between herpesvirus seroprevalence and the development of type 2 diabetes. The authors applied multiplex antibody assays and saw high co-occurrence of herpesviruses in this population-based study, where individuals exhibited antibodies against an average of four out of seven examined herpesviruses. Herpes simplex virus 2 increased the risk of (pre)diabetes incidence by more than 50% and cytomegalovirus by more than 30%. Many other factors such as age increase both the risk for viral infection and the risk for (pre)diabetes development. However, the authors report that the results were robust when they adjusted for potential confounders. The authors conclude that these findings highlight the need to better understand the link between asymptomatic viral infections and metabolic diseases and call for viral prevention strategies, potentially including the development of effective vaccines against herpesviruses.
Lessons from single-cell RNA sequencing of human islets – published online 28/04/2022

Mtaki Ngara, Nils Wierup
Islet dysfunction is a key component of type 2 diabetes but due to the complex cell composition of the islets it has not been easy to understand how each of the five islet cell types is affected by or contributes to disease development. In this issue Ngara and Wierup (https://doi.org/10.1007/s00125-022-05699-1) summarise recent advances in islet biology enabled by single-cell RNA sequencing (scRNAseq). The authors discuss how scRNAseq has generated unprecedented insight into important aspects of islet biology, foremost by uncovering cell-type-specific gene expression in all islet cell populations. The technique has also proven highly useful in the stem cell and development fields. When it comes to identifying type 2 diabetes disease mechanisms, we have not yet seen a major breakthrough. However, the authors conclude that advances in computational methods in combination with larger studies will most likely lead to a leap forward in this area within the near future. The figure from this review is available as a downloadable slide.
Sleep deprivation prevents counterregulatory adaptation to recurrent hypoglycaemia – published online 21/04/2022

Svenja Meyhöfer, Katharina Dembinski, Bernd Schultes, Jan Born, Britta Wilms, Hendrik Lehnert, Manfred Hallschmid, Sebastian M. Meyhöfer
Hypoglycaemia unawareness syndrome due to recurrent hypoglycaemic episodes is a major complication of diabetes treatment. Adaptation of the counterregulatory response to recurrent hypoglycaemia may be considered as a learning process that implicates the formation of neurometabolic memory. Recent epidemiological and experimental findings describe sleep as a relevant factor for metabolic control and may be essential for glucose homeostasis. In this issue, Meyhöfer et al (https://doi.org/10.1007/s00125-022-05702-9) report that, compared with regular sleep, sleep deprivation dampens the adaptation to recurrent hypoglycaemia. They also show that neuroglycopenic symptoms during hypoglycaemia are preserved upon sleep deprivation. The authors conclude that sleep may be a potential modulator of metabolic memory.
The 26RFa (QRFP)/GPR103 neuropeptidergic system in mice relays insulin signalling into the brain to regulate glucose homeostasis – published online 27/04/2022
Mouna El Mehdi, Saloua Takhlidjt, Mélodie Devère, Arnaud Arabo, Marie-Anne Le Solliec, Julie Maucotel, Alexandre Bénani, Emmanuelle Nedelec, Céline Duparc, Benjamin Lefranc, Jérôme Leprince, Youssef Anouar, Gaëtan Prévost, Nicolas Chartrel, Marie Picot
The last two decades provided evidence that the central nervous system contributes significantly to the maintenance of glucose homeostasis in the body. However, the molecular mechanisms and neuronal networks involved in this regulation remain largely unknown. In this issue, El Mehdi et al (https://doi.org/10.1007/s00125-022-05706-5) report that 26RFa (also referred to as pyroglutamilated RFamide peptide [QRFP]), a neuropeptide previously found to be involved in the peripheral regulation of glycaemia and the control of feeding behaviour, improves glucose tolerance in mice when administrated centrally. The authors also show that insulin targets the hypothalamic 26RFa-expressing neurons, increasing the peripheral secretion of insulin in a hyperglycaemic context. The authors conclude that these findings have identified a key relay for the central regulation of glucose metabolism by insulin and an entirely new mechanism contributing to overall glucose homeostasis in the body, which may represent a new target for the treatment of diabetes.
Plasma metabolite profiles related to plant-based diets and the risk of type 2 diabetes – published online 08/04/2022

Fenglei Wang, Megu Y. Baden, Marta Guasch-Ferré, Clemens Wittenbecher, Jun Li, Yanping Li, Yi Wan, Shilpa N. Bhupathiraju, Deirdre K. Tobias, Clary B. Clish, Lorelei A. Mucci, A. Heather Eliassen, Karen H. Costenbader, Elizabeth W. Karlson, Alberto Ascherio, Eric B. Rimm, JoAnn E. Manson, Liming Liang, Frank B. Hu
Plant-based diets, especially healthy plant-based diets that are rich in whole grains, fruits and vegetables, have been associated with a lower risk of type 2 diabetes. However, the plasma metabolite profile underlying this association is not clear. In this issue, Wang and Baden et al (https://doi.org/10.1007/s00125-022-05692-8) report that the plasma metabolite profile of healthy plant-based diets is associated with lower type 2 diabetes risk and could explain part of the beneficial association of the healthy plant-based diet. Furthermore, several plasma metabolites (such as trigonelline, hippurate, isoleucine and a small set of triacylglycerols) were identified as potential mediators of the association between plant-based diets and type 2 diabetes. The authors conclude that the identified plasma metabolite profile could be used to assess the adherence of and metabolic response to a healthy plant-based diet for type 2 diabetes prevention.
Effect of dapagliflozin on kidney and cardiovascular outcomes by baseline KDIGO risk categories: a post hoc analysis of the DAPA-CKD trial – published online 21/04/2022

Simke W. Waijer, Priya Vart, David Z. I. Cherney, Glenn M. Chertow, Niels Jongs, Anna Maria Langkilde, Johannes F. E. Mann, Ofri Mosenzon, John J. V. McMurray, Peter Rossing, Ricardo Correa-Rotter, Bergur V. Stefansson, Robert D. Toto, David C. Wheeler, Hiddo J. L. Heerspink
Higher albuminuria and lower estimated glomerular filtration rate (eGFR) are predictors of kidney failure and cardiovascular events, and are the basis of Kidney Disease Improving Global Outcomes (KDIGO) risk categorisation. It is unknown if dapagliflozin’s clinical benefits are generalisable to different chronic kidney disease (CKD) stages, defined by baseline KDIGO risk. In this issue, Waijer et al (https://doi.org/10.1007/s00125-022-05694-6) report the effect of dapagliflozin versus placebo on kidney and cardiovascular outcomes in patients with CKD categorised by KDIGO risk. Dapagliflozin consistently reduced the risk of kidney and cardiovascular events compared with placebo, across all KDIGO risk categories, in patients with or without type 2 diabetes. The benefit of dapagliflozin in slowing eGFR decline was also similar across KDIGO risk categories, as was dapagliflozin safety. The authors conclude that these findings support use of dapagliflozin in a broad range of patients with CKD who are at risk of progressive kidney and cardiovascular disease, to prevent clinically important outcomes.
Unravelling innervation of pancreatic islets – published online 29/03/2022
Rollie F. Hampton, Maria Jimenez-Gonzales, Sarah A. Stanley
Recent innovations in 3D imaging and targeted neuromodulation have advanced our understanding of pancreatic innervation. In this issue, Hampton and Jimenez-Gonzalez et al (https://doi.org/10.1007/s00125-022-05691-9) summarise recent advances that provide insights into the complex anatomy of pancreatic nerves and their roles in modulating islet hormone release and regulation of glucose metabolism. New imaging technologies provide detailed 3D analyses of pancreatic islet innervation in multiple species and suggest rapid changes in islet nerve structure with metabolic disease. Transgenic and viral approaches now allow unprecedented organ-specific and pathway-specific neuromodulation to assess the functional roles of pancreatic nerves. The authors discuss how these technologies provide an opportunity to advance our understanding of pancreatic innervation, which may identify new approaches to treat metabolic disease. The figures from this review are available as a downloadable slideset.
Mucosal-associated invariant T cells are associated with insulin resistance in childhood obesity, and disrupt insulin signalling via IL-17 – published online 19/03/2022

Ronan Bergin, David Kinlen, Nidhi Kedia-Mehta, Eadaoin Hayes, Féaron C. Cassidy, Declan Cody, Donal O’Shea, Andrew E. Hogan
Insulin resistance is one of the first signs of metabolic dysregulation to manifest in childhood obesity, long before the development of overt metabolic disease. However, the primary drivers of insulin resistance in childhood obesity remain to be elucidated. In this issue, Bergin and Kinlen et al (https://doi.org/10.1007/s00125-022-05682-w) report that an innate T cell subset, the mucosal-associated invariant T (MAIT) cell, is strongly associated with insulin resistance in children with obesity. Furthermore, the authors demonstrate that the production of IL-17 by MAIT cells in particular is associated with insulin resistance. The authors then provide evidence from cell-based models that IL-17 can directly disrupt insulin-mediated glucose uptake. The authors conclude that these findings highlight a novel cellular driver of insulin resistance, which may represent a future therapeutic target.
XBP1 maintains beta cell identity, represses beta-to-alpha cell transdifferentiation and protects against diabetic beta cell failure during metabolic stress in mice – published online 22/03/2022

Kailun Lee, Jeng Yie Chan, Cassandra Liang, Chi Kin Ip, Yan-Chuan Shi, Herbert Herzog, William E. Hughes, Mohammed Bensellam, Viviane Delghingaro-Augusto, Mark E. Koina, Christopher J. Nolan, D. Ross Laybutt
Islet beta cell dedifferentiation has been implicated in beta cell failure in type 2 diabetes, although the mechanisms are poorly defined. The endoplasmic reticulum stress response factor X-box binding protein 1 (XBP1) is a major regulator of the unfolded protein response. Reduced XBP1 expression has been observed in islets of people with type 2 diabetes. In this issue, Lee et al (https://doi.org/10.1007/s00125-022-05669-7) report that XBP1 is crucial for the maintenance of beta cell identity and repression of beta-to-alpha transdifferentiation in mice. The authors show that deletion of Xbp1 in adult mouse beta cells deactivates beta cell identity genes and derepresses beta cell dedifferentiation and alpha cell genes. They also demonstrate that XBP1 is required for beta cell compensation and protection against diabetes in insulin-resistant states. It is proposed that XBP1 protects against beta cell apoptosis during metabolic stress by promoting the beta cell’s antioxidant response. The authors conclude that targeting XBP1 might help to reverse the process of beta cell dedifferentiation and restore functional beta cell mass in type 2 diabetes.
Young-onset diabetes in Asian Indians is associated with lower measured and genetically determined beta cell function – published online 05/03/2022

Moneeza K. Siddiqui, Ranjit Mohan Anjana, Adem Y. Dawed, Cyrielle Martoeau, Sundararajan Srinivasan, Jebarani Saravanan, Sathish K. Madanagopal, Alasdair Taylor, Samira Bell, Abirami Veluchamy, Rajendra Pradeepa, Naveed Sattar, Radha Venkatesan, Colin N. A. Palmer, Ewan R. Pearson, Viswanathan Mohan
South Asians in general, and Asian Indians in particular, are at greater risk of early onset type 2 diabetes than white Europeans. This contributes to the higher prevalence of diabetes in people of South Asian descent and the increasing burden of diabetes in South Asia. In this issue, Siddiqui and Anjana et al (https://doi.org/10.1007/s00125-022-05671-z) use data from non-migrant populations and show that the prevalence of lean young-onset type 2 diabetes is two to four times higher in Asian Indians compared with white Europeans. This phenotype highlights the potential role of poor insulin secretion due to impaired beta cell function in South Asians. The authors applied partitioned polygenic scores (pPS) for poor beta cell function to genetic data from India, Scotland and the UK Biobank, and report that South Asians have a greater genetic burden of beta cell dysfunction. They find that this genetic risk explains, in part, the higher risk of young-onset type 2 diabetes in lean South Asians. The authors conclude that these findings highlight the inter-ethnic differences in the genetics of diabetes and have implications for diabetes care for South Asians.
Incidence of newly diagnosed diabetes after Covid-19 – published online 16/03/2022

Wolfgang Rathmann, Oliver Kuss, Karel Kostev
Inflammation caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may result in insulin resistance. It is unclear whether these metabolic changes are temporary, or if coronavirus disease-2019 (Covid-19) may increase the risk of developing diabetes. In this issue, Rathmann et al (https://link.springer.com/article/10.1007/s00125-022-05670-0) report that adults who recovered from mild Covid-19 had a higher risk of developing type 2 diabetes than a matched control group who had other types of respiratory infections. New cases of type 2 diabetes were more common in individuals who tested positive for Covid-19 in primary care compared with those diagnosed with an acute upper respiratory infection (15.8 vs 12.3 per 1000 people per year, giving an incidence rate ratio of 1.28). The authors conclude that although type 2 diabetes is not likely to be a problem for the majority of people with mild Covid-19, anyone who has recovered from Covid-19 should be aware of symptoms such as fatigue, frequent urination and increased thirst, and promptly seek medical advice.
Maturation of beta cells: lessons from in vivo and in vitro models – published online 04/03/2022

Tom Barsby, Timo Otonkoski
Many recent studies have uncovered novel molecular insights into the functional maturation of beta cells following postnatal development and throughout stem cell-derived islet differentiation. In this issue, Barsby and Otonkoski (https://doi.org/10.1007/s00125-022-05672-y) draw together recent findings in the regulatory and metabolic mechanisms underlying this maturation. The authors discuss how the interplay of nutrient sensitivity, metabolic signatures and circadian regulation are all important facets of functional maturation that regulate (and are regulated by) the transcriptomic state of the beta cell. This review further highlights that beta cell maturation is not a binary process and encompasses processes beyond the acquisition of a beta cell identity and the expression of a subset of particular single marker genes. The figures from this review are available as a downloadable slideset.
Endothelial glycocalyx is damaged in diabetic cardiomyopathy: angiopoietin 1 restores glycocalyx and improves diastolic function in mice – published online 25/02/2022

Yan Qiu, Stanley Buffonge, Raina Ramnath, Sophie Jenner, Sarah Fawaz, Kenton P. Arkill, Chris Neal, Paul Verkade, Stephen J. White, Melanie Hezzell, Andrew H. J. Salmon, M.-Saadeh Suleiman, Gavin I. Welsh, Rebecca R. Foster, Paolo Madeddu, Simon C. Satchell
Diabetic cardiomyopathy is a serious and under-recognised complication of diabetes. The first sign is diastolic dysfunction, which progresses to heart failure. Endothelial glycocalyx plays multiple vital roles in the microcirculation and whilst it is known to be compromised in diabetes, it has not previously been studied in the coronary microcirculation in diabetes. In this issue, Qiu et al (https://doi.org/10.1007/s00125-022-05650-4) report that, in mouse models of diabetes, diastolic dysfunction is associated with glycocalyx loss from coronary microvascular endothelial cells and increased microvascular permeability. The authors also show that endothelial glycocalyx damage is sufficient to impair cardiac function. They provide evidence for increased matrix metalloproteinase activity as a potential mechanism of endothelial glycocalyx damage. They go on to demonstrate that angiopoietin 1 restores the endothelial glycocalyx and ameliorates diastolic dysfunction in diabetes. The authors conclude that these findings identify coronary microvascular endothelial glycocalyx damage as a contributor to the development of diabetic cardiomyopathy and, therefore, as a therapeutic target for heart failure in people with diabetes.
Early DNA methylation changes in children developing beta cell autoimmunity at a young age – published online 10/02/2022

Inna Starskaia, Essi Laajala, Toni Grönroos, Taina Härkönen, Sini Junttila, Roosa Kattelus, Henna Kallionpää, Asta Laiho, Veronika Suni, Vallo Tillmann, Riikka Lund, Laura L. Elo, Harri Lähdesmäki, Mikael Knip, Ubaid Ullah Kalim, Riitta Lahesmaa
DNA methylation changes associated with type 1 diabetes were previously detected in individuals clinically diagnosed with the disease. Recently, using array-based methods, these changes were also detected in whole blood samples from individuals before they developed diabetes. In this issue, Starskaia et al (https://doi.org/10.1007/s00125-022-05657-x) use genome-wide reduced representation bisulphite sequencing to detect cell type-specific DNA methylation changes associated with type 1 diabetes before clinical diagnosis, and even before the appearance of autoantibodies. The authors conclude that the early epigenetic changes associated with type 1 diabetes identified in this study may contribute to pathogenesis and provide basis for the early detection of diabetes.
Spatial and transcriptional heterogeneity of pancreatic beta cell neogenesis revealed by a time-resolved reporter system – published online 03/03/2022

Shugo Sasaki, Michelle Y. Y. Lee, Yuka Wakabayashi, Luka Suzuki, Helena Winata, Miwa Himuro, Taka-aki Matsuoka, Iichiro Shimomura, Hirotaka Watada, Francis C. Lynn, Takeshi Miyatsuka
Although endocrine pancreas development has been investigated by many researchers, the beta cell developmental niche, or precisely where and when beta cells arise in vivo, remains less well described. Part of the reason for this is that there have been no methods to readily detect newly generated beta cells in situ. In this issue, Sasaki et al (https://doi.org/10.1007/s00125-022-05662-0) describe a novel time-resolved mouse model, which was developed to distinguish newborn beta cells from more differentiated beta cells. The authors report that this model provides the first in vivo evidence that beta cells arise from two distinct regions: ductal or blood vessel niches. Using this model, the authors also show that single-cell transcriptional heterogeneity during beta cell genesis correlates with the spatial heterogeneity. Furthermore, single-cell mRNA profiles of human embryonic stem cell-derived beta-like cells demonstrated a transcriptional similarity with the data from newborn beta cells in mice. The authors conclude that this work provides insight for the future development of regenerative therapies for diabetes.
Epigenome-wide association study of incident type 2 diabetes: a meta-analysis of five prospective European cohorts – published online 15/02/2022
Eliza Fraszczyk, Annemieke M. W. Spijkerman, Yan Zhang, Stefan Brandmaier, Felix R. Day, Li Zhou, Paul Wackers, Martijn E. T. Dollé, Vincent W. Bloks, Xīn Gào, Christian Gieger, Jaspal Kooner, Jennifer Kriebel, H. Susan J. Picavet, Wolfgang Rathmann, Ben Schöttker, Marie Loh, W. M. Monique Verschuren, Jana V. van Vliet-Ostaptchouk, Nicholas J. Wareham, John C. Chambers, Ken K. Ong, Harald Grallert, Hermann Brenner, Mirjam Luijten, Harold Snieder
Epigenetics may play a role in the development of type 2 diabetes, and predictive DNA methylation markers have been identified in single-cohort epigenome-wide association studies. Combining results from several prospective cohorts may identify additional markers. In this issue, Fraszczyk et al (https://doi.org/10.1007/s00125-022-05652-2) report 76 DNA methylation markers from the meta-analysis of five European cohorts, of which 63 were novel for incident type 2 diabetes. The authors suggest that epigenetics has the potential to elucidate new biological pathways underlying the development of type 2 diabetes, and predictive DNA methylation markers could ultimately be useful in type 2 diabetes prevention efforts.
When therapeutic drugs lead to diabetes – published online 04/03/2022

Bruno Fève and André J. Scheen
Drug-induced diabetes is not a novel concern, and this is examined in this issue by Fève and Scheen (https://doi.org/10.1007/s00125-022-05666-w). The archetype of this iatrogenic complication is glucocorticoid-induced diabetes, which remains the most frequently encountered one in clinical practice. However, we should not overlook the high prevalence of diabetes caused by antipsychotics, in particular by second generation compounds. The pharmacopoeia of antiretrovirals has greatly improved since the first classes of these drugs became available at the end of the 1980s; however, there is still a residual metabolic toxicity of several new generation molecules. Even more recently, the advent of immunotherapies in oncology has been accompanied by the emergence of diabetes cases that are reminiscent of the phenotype of type 1 diabetes. This short review is focused on these four families of diabetogenic drugs, and will provide information on the prevalence of this complication, the main clinical presentations and the key pathophysiological mechanisms, before addressing the management and prevention of these chemically induced forms of diabetes. The figure from this review is available as a downloadable slide.
The influence of bright and dim light on substrate metabolism, energy expenditure and thermoregulation in insulin-resistant individuals depends on time of day – published online 02/02/2022
Jan-Frieder Harmsen, Jakob Wefers, Daniel Doligkeit, Luc Schlangen, Bas Dautzenberg, Pascal Rense, Dirk van Moorsel, Joris Hoeks, Esther Moonen-Kornips, Marijke C. M. Gordijn, Wouter D. van Marken Lichtenbelt, Patrick Schrauwen
We spend most of our time indoors under light conditions that are either not as bright as natural daylight or too bright in the evening after sunset. Such suboptimal light conditions are considered to be risk factors for metabolic diseases, with detrimental effects of light exposure at night on sleep quality and glucose metabolism. In this issue, Harmsen and Wefers et al (https://doi.org/10.1007/s00125-021-05643-9) investigate the metabolic impact in insulin-resistant men and women of a 24h light scheme resembling the natural light/dark cycle, with bright light during daytime and dim light during the evening. They report that the optimised light scheme was beneficial for plasma glucose levels preceding dinner, energy expenditure during the night, and diurnal rhythms in peripheral skin temperature. The authors conclude that these findings provide the rationale to further explore indoor lighting designs to prevent or treat metabolic diseases.
Glucolipotoxicity promotes the capacity of the glycerolipid/NEFA cycle supporting the secretory response of pancreatic beta cells – published online 12/01/2022

Lucie Oberhauser, Cecilia Jiménez-Sánchez, Jesper Grud Skat Madsen, Dominique Duhamel, Susanne Mandrup, Thierry Brun, Pierre Maechler
About three decades ago, in the context of type 2 diabetes, the concept of lipotoxicity, and later of glucolipotoxicity, was applied to pancreatic beta cells. However, after all these years it remains debated whether essential components of the organ’s chemistry, namely fat and sugar, could be qualified as genuine toxic molecules. In this issue, Oberhauser et al (https://doi.org/10.1007/s00125-021-05633-x) report results from a study in which they exposed pancreatic islets to various so-called glucolipotoxic culture conditions before analysing their response to standard conditions of glucose-stimulated insulin secretion. The authors report that high glucose, rather than glucose per se, is detrimental for beta cell function. Cells exposed to fatty acids and high glucose exhibited massive fat storage, which was rapidly mobilised upon return to normal conditions. Such fat turnover was instrumental for the preservation of the secretory response in cells experiencing glucotoxicity. The authors conclude that these findings advocate against continuous energy-rich snacking without fasting periods for the preservation of beta cell function.
Understanding the pathogenesis of lean non-autoimmune diabetes in an African population with newly diagnosed diabetes – published online 09/02/2022

Davis Kibirige, Isaac Sekitoleko, William Lumu, Angus G. Jones, Andrew T. Hattersley, Liam Smeeth, Moffat J. Nyirenda
Atypical diabetes has been described in sub-Saharan Africa, with non-insulin-requiring apparent type 2 diabetes seen in lean and sometimes young individuals. However, robust data on the clinical and metabolic characterisation of these lean individuals with diabetes are lacking. In this issue, Kibirige et al (https://doi.org/10.1007/s00125-021-05644-8) investigated the phenotype of newly diagnosed adult-onset diabetes in Uganda. The authors report that individuals with a lean type 2 diabetes phenotype were predominantly male, exhibiting significant pancreatic beta cell dysfunction but no evidence of the metabolic syndrome or insulin resistance. This study further adds to evidence of differences in the pathogenesis of type 2 diabetes across populations. The authors suggest that due to the observed differences in underlying pathophysiological defects of type 2 diabetes, there is a need for interventional studies to investigate the optimal individualised therapies for individuals with a lean type 2 diabetes phenotype in sub-Saharan Africa.
Effectiveness of continuous glucose monitoring in maintaining glycaemic control among people with type 1 diabetes mellitus: a systematic review of randomised controlled trials and meta-analysis – published online 09/02/2022

Evelyn Teo, Norasyikin Hassan, Wilson Tam, Serena Koh
Glucose monitoring is a key method for individuals with type 1 diabetes to maintain adequate glycaemic control and delay the onset of diabetic complications. The limitations of traditional self-monitoring of blood glucose (SMBG) can be overcome by using continuous glucose monitoring (CGM). Current reviews regarding the effectiveness of CGM and SMBG on glycaemic control revealed several research gaps. In this issue, Teo et al (https://doi.org/10.1007/s00125-021-05648-4) report that CGM significantly reduces HbA1c levels compared with SMBG, with larger effects observed among those with higher baseline HbA1c. However, their results show that CGM has no effect on severe hypoglycaemia and diabetic ketoacidosis. The authors conclude that CGM is superior to SMBG in improving glycaemic control, especially among those with poorly controlled glycaemia. They also suggest that individuals with poorly controlled glycaemia would benefit most from CGM compared with SMBG in the community.
Lipids, hyperreflective crystalline deposits and diabetic retinopathy: potential systemic and retinal-specific effect of lipid-lowering therapies – published online 11/02/2022

Alicia J. Jenkins, Maria B. Grant, Julia V. Busik
The highly metabolically active retina obtains essential lipids through both endogenous biosynthesis and via the systemic circulation. Both quantitative and qualitative changes in lipids have been associated with diabetic retinopathy. Whilst the role of lipids and lipid-modifying drugs in cardiovascular disease in people with diabetes is well-studied, their roles in diabetic retinopathy are currently less well known. In this issue, Jenkins et al (https://doi.org/10.1007/s00125-022-05655-z) review the potential role of lipids and lipid-lowering drugs in diabetic retinopathy, examining results from retinal tissue analyses, clinical observational studies, clinical trials and meta-analyses. The authors discuss several statin and fibrate trials that were designed to predominantly address cardiovascular outcomes, but which have also reported potential retinal benefits. They outline the many challenges in this clinically important field, but also highlight that ongoing research in this area. This includes several in-progress trials of lipid drugs that have diabetic retinopathy-related primary endpoints, which may further elucidate the potential mechanisms by which lipid-modifying therapies could impact diabetic retinopathy. The figures from this review are available as a downloadable slideset.
Wt1 haploinsufficiency induces browning of epididymal fat and alleviates metabolic dysfunction in mice on high-fat diet – published online 30/11/2021

Karin M. Kirschner, Anna Foryst-Ludwig, Sabrina Gohlke, Chen Li, Roberto E. Flores, Ulrich Kintscher, Michael Schupp, Tim J. Schulz, Holger Scholz
The induction of thermogenically active beige adipocytes in white adipose tissue (WAT) is a key feature of WAT browning. Browning has recently gained interest for its potential use to enhance energy expenditure. Beige adipocytes can be readily induced in subcutaneous WAT of mice, whereas visceral WAT is more resistant to browning. In this issue, Kirschner et al (https://doi.org/10.1007/s00125-021-05621-1) show that otherwise healthy mice, heterozygous for the Wilms tumour gene, Wt1, display morphological and genetic signs of browning in their visceral WAT. Strikingly, Wt1 heterozygosity improved whole-body glucose tolerance and prevented severe hepatic steatosis under a high-fat diet. Mechanistically, the authors identified WT1 as an upstream regulator of Aldh1a1 and Zfp423, key suppressors of the thermogenic programme in adipocytes. Their data provide evidence that WT1 downregulates thermogenic genes and functions as a white adipocyte determination factor in visceral WAT. The authors conclude that targeting Wt1 expression in visceral fat may offer a promising novel approach to fight metabolic disorders.
Dietary carbohydrate restriction augments weight loss-induced improvements in glycaemic control and liver fat in individuals with type 2 diabetes: a randomised controlled trial – published online 07/01/2022

Mads N. Thomsen, Mads J. Skytte, Amirsalar Samkani, Martin H. Carl, Philip Weber, Arne Astrup, Elizaveta Chabanova, Mogens Fenger, Jan Frystyk, Bolette Hartmann, Jens J. Holst, Thomas M. Larsen, Sten Madsbad, Faidon Magkos, Henrik S. Thomsen, Steen B. Haugaard, Thure Krarup
Weight loss is the cornerstone of management of type 2 diabetes. Whether a diet reduced in carbohydrate and increased in protein and fat can augment the beneficial effects of weight loss, compared with a conventional diabetes diet, is not known. In this issue, Thomsen et al (https://doi.org/10.1007/s00125-021-05628-8) present the results of a randomised study that evaluated the metabolic effects of matched 6% weight loss, induced after 6 weeks of a fully-provided carbohydrate-reduced diet (30% of energy from carbohydrate) or a conventional diabetes diet (50% of energy from carbohydrate) in individuals with type 2 diabetes. The authors show that the experimental diet was well tolerated and, for the same amount of weight loss as that induced by the control diet, augmented the reduction in HbA1c by 1.9 mmol/mol, in liver fat by 26%, in plasma triacylglycerol by 18% and in diurnal blood glucose by 0.8 mmol/l. The authors conclude that carbohydrate reduction has weight loss-independent beneficial metabolic effects and should be considered in the treatment of type 2 diabetes.
Multi-ethnic GWAS and fine-mapping of glycaemic traits identify novel loci in the PAGE Study – published online 24/12/2021

Carolina G. Downie, Sofia F. Dimos, Stephanie A. Bien, Yao Hu, Burcu F. Darst, Linda M. Polfus, Yujie Wang, Genevieve L. Wojcik, Ran Tao, Laura M. Raffield, Nicole D. Armstrong, Hannah G. Polikowsky, Jennifer E. Below, Adolfo Correa, Marguerite R. Irvin, Laura J. F. Rasmussen-Torvik, Christopher S. Carlson, Lawrence S. Phillips, Simin Liu, James S. Pankow, Stephen S. Rich, Jerome I. Rotter, Steven Buyske, Tara C. Matise, Kari E. North, Christy L. Avery, Christopher A. Haiman, Ruth J. F. Loos, Charles Kooperberg, Mariaelisa Graff, Heather M. Highland
Previous genome-wide association studies (GWAS) have identified over 500 loci associated with type 2 diabetes and glycaemic-related traits. However, most of these studies were conducted in populations of European ancestry. This lack of ancestral diversity hinders efforts to identify novel loci, refine causal signals through fine-mapping, and develop equitable genetic approaches for precision medicine and risk prediction. In this issue, Downie et al (https://doi.org/10.1007/s00125-021-05635-9) identify novel glycaemic trait loci in the ancestrally diverse Population Architecture using Genomics and Epidemiology (PAGE) Study. Specifically, the authors identify three fasting insulin novel loci in a transethnic meta-analysis, one novel low-frequency fasting glucose locus in an African American-specific analysis, and novel independent secondary signals at known fasting glucose and insulin loci. The authors conclude that these findings highlight the continued importance of conducting genetic studies in diverse populations and provide new insights into the genetic architecture of glycaemic traits.
Artificial intelligence utilising corneal confocal microscopy for the diagnosis of peripheral neuropathy in diabetes mellitus and prediabetes – published online 21/11/2021

Frank G. Preston, Yanda Meng, Jamie Burgess, Maryam Ferdousi, Shazli Azmi, Ioannis N. Petropoulos, Stephen Kaye, Rayaz A. Malik, Yalin Zheng, Uazman Alam
The accurate detection of diabetic neuropathy in routine clinical practice remains a major unmet clinical need. Current screening practices largely rely on insensitive tests which only primarily detect the insensate foot. It has been demonstrated that artificial intelligence (AI) trained using annotated corneal confocal microscopy images can provide accurate segmentation of corneal nerve images, allowing the detection of peripheral neuropathy. In this issue, Preston and Meng et al (https://doi.org/10.1007/s00125-021-05617-x) report that AI utilising corneal nerve images can accurately classify peripheral neuropathy in people with prediabetes and diabetes, without the need for underlying nerve segmentation. This was achieved by use of a single corneal confocal microscope image. The authors discuss that, as annotation of the image/dataset was not required, larger sets of unannotated images may be leveraged in providing a more robust model. The authors conclude that with validation in a larger real-world study, the AI algorithm has considerable potential for adoption into screening programmes for diabetic neuropathy.
Metabolic, structural and biochemical changes in diabetes and the development of heart failure – published online 07/01/2022
Kim L. Ho, Qutuba G. Karwi, David Connolly, Simran Pherwani, Ezra B. Ketema, John R. Ussher, Gary D. Lopaschuk
Diabetes increases the risk of heart failure by over two-fold, and the need to improve our understanding of the way in which this occurs is becoming ever important. In this issue, Ho et al (https://doi.org/10.1007/s00125-021-05637-7) summarise the cardiac metabolic, structural, and biochemical changes that occur in diabetes. The authors discuss how hyperlipidaemia and hyperglycaemia contribute to changes in fatty acid and glucose metabolism, and that structural remodelling of the heart occurs in the form of hypertrophy and fibrosis. They go on to explore how, biochemically, impairments in calcium handling, glucotoxicity, lipotoxicity, and transcriptional and translational modifications all contribute to cardiac dysfunction in diabetes. The authors conclude by highlighting that with the increasing amount of research going into the mechanisms by which certain diabetes drugs improve cardiovascular outcomes, new therapeutic strategies are emerging to treat diabetes and heart failure. The figures from this review are available as a downloadable slideset.
Upregulation of HLA class II in pancreatic beta cells from organ donors with type 1 diabetes – published online 21/12/2021

Estefania Quesada-Masachs, Samuel Zilberman, Sakthi Rajendran, Tiffany Chu, Sara McArdle, William B. Kiosses, Jae-Hyun M. Lee, Burcak Yesildag, Mehdi A. Benkahla, Agnieszka Pawlowska, Madeleine Graef, Susanne Pfeiffer, Zbigniew Mikulski, Matthias von Herrath
For many decades, the question of whether HLA class II can be expressed by pancreatic beta cells has been controversial. In this issue, Quesada-Masachs et al (https://doi.org/10.1007/s00125-021-05619-9) report that HLA class II is upregulated in islets of pancreatic tissue sections from organ donors with type 1 diabetes: 28% of the beta cells from the patients with type 1 diabetes expressed HLA class II. Immunofluorescence microscopy was used to thoroughly quantify HLA class II in situ, using a machine learning approach. Furthermore, the authors report that healthy human islets stimulated with proinflammatory cytokines upregulate HLA class I and class II, as measured by quantitative fluorescent microscopy and RNA sequencing. The authors suggest that a crosstalk could exist between beta cells and CD4+ T cells in type 1 diabetes, although further research is necessary to demonstrate this cellular communication and elucidate its biological role in disease initiation and progression.
Relative leucocyte telomere length is associated with incident end-stage kidney disease and rapid decline of kidney function in type 2 diabetes: analysis from the Hong Kong Diabetes Register – published online 22/11/2021

Feifei Cheng, Andrea O. Luk, Hongjiang Wu, Claudia H. T. Tam, Cadmon K. P. Lim, Baoqi Fan, Guozhi Jiang, Luke Carroll, Aimin Yang, Eric S. H. Lau, Alex C. W. Ng, Heung Man Lee, Elaine Chow, Alice P. S. Kong, Anthony C. Keech, Mugdha V. Joglekar, Wing Yee So, Anandwardhan A. Hardikar, Juliana C. N. Chan, Alicia J. Jenkins, Ronald C. W. Ma
Telomere length shortening, representing reduction in the protective caps at the ends of our chromosomes, is known to be associated with biological ageing and different cardiometabolic diseases, although it is unclear whether it has prognostic significance for predicting kidney disease in diabetes. In this issue, Cheng et al (https://doi.org/10.1007/s00125-021-05613-1) report that in a large cohort of people with type 2 diabetes from Hong Kong, reduced telomere length in white blood cells was an independent predictor for decline in kidney function and future risk of kidney failure. The authors suggest that this effect was independent of other established risk factors for kidney dysfunction, and improves prediction beyond that provided by clinical factors alone. The authors conclude that these findings indicate telomere length shortening may be helpful in stratifying the future risk of kidney disease in people with diabetes.
Impact of insufficient sleep on dysregulated blood glucose control under standardised meal conditions – published online 30/11/2021

Neli Tsereteli, Raphael Vallat, Juan Fernandez-Tajes, Linda M. Delahanty, Jose M. Ordovas, David A. Drew, Ana M. Valdes, Nicola Segata, Andrew T. Chan, Jonathan Wolf, Sarah E. Berry, Matthew P. Walker, Timothy D. Spector, Paul W. Franks
Small in-patient studies and larger observational studies suggest that features of how we sleep may affect our metabolic health. In this issue, Tsereteli et al (https://doi.org/10.1007/s00125-021-05608-y) report data from the largest experimental study to date focusing on objectively assessed sleep and its impact on postprandial blood glucose following standardised breakfast meals. The authors show that poor sleep efficiency and later bedtime routines worsen blood glucose responses overall. The authors also show that at an individual level, sleep matters, as person-specific deviations from normal sleep patterns also impact the blood glucose response to breakfast. This was especially true when an oral glucose load was given as the breakfast meal, suggesting that the popular practice of consuming energy drinks after a poor night’s sleep may be particularly detrimental for blood glucose regulation. The authors conclude that these findings underscore the importance of sleep in the optimal regulation of human metabolic health.
Environmental risk factors of type 2 diabetes—an exposome approach – published online 18/11/2021
Joline W. J. Beulens, Maria G. M. Pinho, Taymara C. Abreu, Nicole R. den Braver, Thao M. Lam, Anke Huss, Jelle Vlaanderen, Tabea Sonnenschein, Noreen Z. Siddiqui, Zhendong Yuan, Jules Kerckhoffs, Alexandra Zhernakova, Milla F. Brandao Gois, Roel C. H. Vermeulen
A major part of the burden of type 2 diabetes is attributed to environmental risks and modifiable risk factors such as lifestyle. The environment we live in, and changes to it, can therefore contribute substantially to the prevention of type 2 diabetes at a population level. In this issue, Beulens et al (https://doi.org/10.1007/s00125-021-05618-w) summarise the evidence on the role of the food-, built-, physico-chemical- and social environment in the development of type 2 diabetes. The authors discuss the established associations of air pollution, residential noise and area-level socioeconomic deprivation with an increased risk of type 2 diabetes, and highlight that neighbourhood walkability and green space are associated with a reduced risk of type 2 diabetes. The contribution of the food environment, along with other aspects of the social environment and outdoor temperature are less clear. The authors suggest that these environmental factors affect type 2 diabetes risk mainly through mechanisms that incorporate lifestyle factors, the microbiome, inflammation or chronic stress. The figures from this review are available as a downloadable slideset
Lipotoxicity-induced circGlis3 impairs beta cell function and is transmitted by exosomes to promote islet endothelial cell dysfunction – published online 09/11/2021
Li Xiong, Li Chen, Liting Wu, Weiman He, Dubo Chen, Zishan Peng, Jin Li, Xiaonan Zhu, Lei Su, Yanbing Li, Yingying Gong, Haipeng Xiao
Circular RNAs (circRNAs) play important roles in regulating beta cell function, and exosomes are essential mediators of intercellular communication. However, the role of exosomal circRNAs in type 2 diabetes is poorly understood. In this issue, Xiong et al (https://doi.org/10.1007/s00125-021-05591-4) report that circGlis3 (Gli-similar 3) participates in the development of type 2 diabetes in two different ways. In a conventional way, circGlis3 exerts deleterious effects on beta cells by inhibiting cell survival and insulin secretion. In an unconventional way, by acting as a mediator of intercellular crosstalk, beta cell-derived exosomal circGlis3 promotes islet endothelial cell dysfunction through the glucocorticoid modulatory element-binding protein 1 (GMEB1)/ heat shock protein 27 (HSP27) signalling pathway. They also demonstrate that exosomal circGlis3 is upregulated by lipotoxicity and is found at higher levels in mouse models of diabetes and in the serum of participants with type 2 diabetes. The authors conclude that this study provides new insights into the pathogenesis of type 2 diabetes and suggests the significance of circGlis3 as a potential biomarker and therapeutic target for the disease.
Interpreting global trends in type 2 diabetes complications and mortality – published online 27/11/2021
Mohammed K. Ali, Jonathan Pearson-Stuttard, Elizabeth Selvin, Edward W. Gregg
Trends in diabetes complications and mortality rates convey the health impacts of diabetes and serve as a barometer of whether clinical practice, intervention programmes and policies are achieving their intended goals. In this issue, Ali et al (https://doi.org/10.1007/s00125-021-05585-2) review recent published data to characterise patterns in type 2 diabetes complications and mortality in adults since 2015, noting stark disparities between different populations. For example, while the burden of diabetes in high-income countries is declining, complications and mortality rates are increasing in low- and middle-income countries. Ali and colleagues discuss how data sources and definitions may be influencing rates and trends observed, and recommend four critical areas of investment to harmonise and bridge the data divide: (1) increasing investments in data collection systems; (2) standardising case definitions and approaches to ascertainment; (3) strengthening analytical capacity; and (4) developing and implementing structured guidelines for reporting of data.