Instructions to Authors
- General information
-
Diabetologia publishes original clinical, translational and experimental research on all aspects of diabetes research and related subjects, provided they have scientific merit and represent an important advance in knowledge. There is no submission fee or page charges. Manuscripts must be written in English. Diabetologia does not insist that authors follow journal guidelines in terms of length of article or formatting at the point of submission but authors of overlength articles will be asked to reduce the word count at the revision stage if their article survives peer-review. A ‘Research in context’ summary is required at the point of submission, but a graphical abstract will be requested only if an article survives peer-review.
Please note that the journal does not consider presubmission enquiries. Instead it operates a rapid triage system whereby manuscripts are assessed by the Editorial Board and any that are not fully competitive for publication are returned very quickly.
Prior to acceptance, all papers are screened to check for plagiarism and image manipulation. Diabetologia expects authors of accepted manuscripts to keep original data available for a minimum of 5 years.
Artificial intelligence (AI)-assisted technologies
Authors must disclose, both in their manuscript and cover letter, any use of artificial intelligence (AI)-assisted technologies (such as Large Language Models [LLMs], chatbots, or image creators). Chatbots (such as ChatGPT) should not be listed as authors because they cannot be responsible for the accuracy, integrity and originality of the work, and these responsibilities are required for authorship (see Authorship criteria). Therefore, humans are responsible for any submitted material that includes the use of AI-assisted technologies. Authors should carefully review and edit the result because AI can generate authoritative-sounding output that can be incorrect, incomplete or biased. Authors should not list AI and AI-assisted technologies as an author or co-author, nor cite AI as an author. Authors should be able to assert that there is no plagiarism in their paper, including in text and images produced by the AI. Humans must ensure there is appropriate attribution of all quoted material, including full citations.
- Types of articles
-
Original articles
A maximum of 4000 words in the main text plus up to 50 references. The abstract, references, tables and figure legends are excluded from the word count, as are acknowledgements and other end matter. Please note that slightly overlength manuscripts will still be considered however authors will be asked to shorten their paper if the article survives peer review. Authors of manuscripts that are significantly overlength may be asked to reduce the number of words prior to their paper going for peer review. Please go to Organisation and content of papers below for information on structuring your article.
Short communications
A maximum of 1500 words in the main text plus approximately ten references and normally no more than two illustrations (tables or figures or one of each). Please go to Organisation and content of papers below for information on structuring your article.
Extended articles
Diabetologia welcomes pre-submission enquiries from authors interested in submitting extended articles to the journal. Extended articles will have a maximum of 8000 words in the main text, up to ten figures plus up to 100 references. The abstract, references, tables and figure legends are excluded from the word count, as are acknowledgements and other end matter. Pre-submission inquiries by e-mail to the editorial office (diabetologia-j@bristol.ac.uk) are mandatory and should include the abstract of the paper and a brief explanation of why the work requires the format of an extended article. Please use ‘presubmission enquiry: extended article’ as the subject line of your email. As for all articles, the threshold for acceptance will be very high.
Systematic reviews
A maximum of 4000 words in the main text with unlimited references. Authors must register their study in a publicly accessible database (e.g. PROSPERO, Open Science Framework, Research Registry) and include the registration number in the manuscript. Diabetologia may, in the future, refuse to consider systematic reviews that have been registered after data extraction has begun. The study protocol should be submitted as supplementary material.
There is no need to contact the Editor-in-Chief before submitting a systematic review; please upload at https://mc.manuscriptcentral.com/diabetologia in the usual way, along with a PRISMA or MOOSE checklist. See Reporting guidelines.
Meta-analyses
A maximum of 4000 words in the main text with unlimited references. We recommend that authors register their study in a publicly accessible database and submit the study protocol as supplementary material.
There is no need to contact the Editor-in-Chief before submitting a meta-analysis; please upload at https://mc.manuscriptcentral.com/diabetologia in the usual way.
For meta-analyses of randomised controlled trials, follow PRISMA reporting guidelines – include a flow diagram in your manuscript and submit a completed PRISMA checklist.
For meta-analyses of observational studies in epidemiology, follow either PRISMA reporting guidelines or MOOSE reporting guidelines and submit a completed PRISMA or MOOSE checklist.
Umbrella reviews
A maximum of 4000 words in the main text with unlimited references. As for systematic reviews, authors of umbrella reviews must register their study in a publicly accessible database (e.g. PROSPERO, Open Science Framework, Research Registry) and include the registration number in the manuscript. The study protocol should be submitted as supplementary material.
There is no need to contact the Editor-in-Chief before submitting an umbrella review; please upload at https://mc.manuscriptcentral.com/diabetologia in the usual way, along with a PRISMA or MOOSE checklist. See Reporting guidelines.Reviews
Reviews for Diabetologia are commissioned directly, and we no longer routinely consider unsolicited reviews for publication.
For debate
A maximum of 2500 words in the main text plus up to 40 references and two illustrations (tables or figures or one of each). These will normally be solicited by the Editor-in-Chief although unsolicited articles will be considered.
Letters to the editor
A maximum of 1000 words, plus 8 references and normally no more than one table or one figure. Letters are the forum for either: (1) Correspondence – comments with critical assessment of papers recently published in Diabetologia which, at the Editor-in-Chief’s discretion, will be sent to the authors of the original paper for comment and both letter and reply published together; or (2) Research letters – observations providing concise and important new information. Research letters are formatted as letters, i.e. in individual paragraphs with no headings and no abstract.
The lead authors of Letter-responses are responsible for contacting all authors of the original paper to ascertain whether they wish to be included in the reply.Authors whose papers exceed permitted word counts at the point of submission will be asked to shorten their paper if the article survives peer review.
-
(Co-)authorship is based upon the following conditions:
1) Each author has participated sufficiently in the work represented by the article to take public responsibility for the content. Participation has included (i) conception or design of the work; or the acquisition, analysis or interpretation of data for the work; and (ii) drafting the article or reviewing it critically for important intellectual content; and (iii) final approval of the version to be published.
2) Authorship has not been justified solely by collection of data, or other evidence, or by obtaining financial resources.
3) Each part or the content of an article to its main conclusions and each step in the work that led to the publication is attributable to at least one author.
4) Persons who have contributed intellectually to the article but whose contributions do not justify authorship are named and their particular contribution described. Such persons have given their permission to be named.
5) The given sequence of authors is the consensus of all contributors.
Chatbots (such as ChatGPT) cannot be listed as authors because they cannot be responsible for the accuracy, integrity and originality of the work, and these responsibilities are required for authorship.
- Reporting guidelines
-
In an attempt to improve transparency in reporting, Diabetologia requires authors to complete the reporting checklist most relevant to their study and to include the checklist items in their manuscript. Please give the relevant page numbers for each item in the list; mark those not applicable to your study as ‘NA’ or leave blank.
The following flowchart will help authors identify the most appropriate checklist for their study:
Reporting guidelines flowchart (click to enlarge)
Preclinical studies
Studies in animal models, cells, islets and other in vitro or ex vivo models should be accompanied by our Preclinical checklist, which is adapted from the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines and the NIH Principles and guidelines for reporting preclinical research. If your study mainly involves human participants, please do not use this checklist, but select the most appropriate checklist from those below.
Human islets
Authors of papers reporting data obtained from studies on human islets should report critical characteristics of the human islets used for research (see checklist). Please note that you will also need to complete either a preclinical or other appropriate checklist
Randomised controlled trials
Reports of randomised controlled trials (RCTs) should include the checklist items set out in the CONSORT guidelines, as well as a patient flow diagram. Authors must submit a completed CONSORT 2010 checklist, along with the original trial protocol (including statistical analyses undertaken). For reports of crossover trials, please use the CONSORT 2019 extension for crossover trials. For reports of non-pharmacological treatment interventions, please use the extension for non-pharmacologic treatments. Other CONSORT extensions can be used as appropriate.
Observational studies
Reports of observational studies (cohort, case-control or cross-sectional designs), non-randomised clinical studies and human interventional studies should include the items detailed in the STROBE checklist. Authors must submit a completed checklist and, if the study protocol is available, this should be uploaded as a supplemental file. STROBE extensions, such as the STREGA extension for genetic association studies, can also be submitted as an alternative where these are more appropriate.
Mendelian randomisation studies
Reports of Mendelian randomisation studies should include the items detailed in the STROBE-MR checklist, and authors must submit a completed checklist which can be downloaded from www.strobe-mr.org/
GWAS studies and studies involving GWAS data
Please see our guidelines for genome-wide association (GWAS) studies and studies involving GWAS data and ensure all appropriate points are included in your study. Please also complete a STREGA or STROBE-MR checklist, as appropriate.
Systematic reviews, umbrella reviews and meta-analyses
Authors of systematic reviews and umbrella reviews must register their study in a publicly accessible database (e.g. PROSPERO, Open Science Framework, Research Registry) and include the registration number in the manuscript. The study protocol should be submitted as supplementary material.
In addition, for systematic reviews, umbrella reviews or meta-analyses of randomised controlled trials, follow PRISMA reporting guidelines and submit a completed PRISMA checklist. For systematic reviews, umbrella reviews or meta-analyses of observational studies in epidemiology, follow either PRISMA reporting guidelines or MOOSE reporting guidelines and submit a completed PRISMA or MOOSE checklist. PRISMA or MOOSE extension checklists can also be submitted if these are more appropriate for your study.
Database studies
For studies that involve the use of patient data from databases, or routinely collected health data, authors should complete either a STROBE checklist or a RECORD checklist. Where data is collected for a more-or-less specific research purpose, STROBE is the most appropriate, whereas RECORD is more suitable for routinely collected data.
Secondary analyses
For secondary analyses of RCTs or observational studies, please complete either a CONSORT or a STROBE checklist, as appropriate. Reference can be made in the checklist and the current paper to previous publications that describe the study in more detail. Any sections of the checklist that do not apply to the current study can be marked ‘not applicable’ (NA). Please note that a STROBE checklist might be more suitable where a cohort from a previous RCT is used to answer a different research question.
Qualitative research
Studies that report qualitative research should include the items detailed in the SRQR checklist and authors must submit a completed checklist.
Mediation analyses
For studies that report primary or secondary mediation analyses of RCTs or observational studies, please complete the AGReMA or AGReMA short form checklist, which can be downloaded from www.agrema-statement.org. For studies that report mediation analyses as the primary focus, the full AGReMA checklist is the most appropriate, whereas the AGReMA short form checklist is more appropriate for studies that report secondary mediation analyses within a primary report of an RCT or observational study.
- Organisation and content of papers
-
Diabetologia does not insist that authors follow journal guidelines in terms of length of article or formatting at the point of submission but authors of overlength articles will be asked to reduce the word count if their article survives peer-review and accepted articles will be brought into line with journal style by copyeditors.
Reporting checklist
Diabetologia insists on submission of a completed checklist alongside a manuscript in an attempt to ensure that the study is reported accurately and thoroughly. See Reporting guidelines.
Title page
This page should state:
- Title of paper (including animal species if appropriate);
- Authors’ names (given name, initials and family name – no qualifications) – please add a note if there are co-first authors;
- Institution(s) of origin;
- ORCID identifiers where possible;
- Corresponding author and email address (co-corresponding authors are permitted, please ensure this is clear);
- Word count (excluding abstract, research in context, references, acknowledgements, funding statement, authors’ relationships and activities, contribution statement, tables and figure legends).
Abstract
Abstracts should be structured into four paragraphs as follows: (1) Aims/hypothesis; (2) Methods; (3) Results; (4) Conclusions/interpretation. The Abstract should contain data to support the main results of your paper. Please do not include unexplained abbreviations. There is no upper word limit.
Please include numerical data in your abstract to support the main findings of your paper, if appropriate. (Our word limit for abstracts is flexible.) Please make sure the data in your abstract can also be found easily in the Results section of your paper or in the tables, and make sure that data are reported consistently in the Abstract and Results, and to the same number of decimal places.
For clinical trials, the trial registry number should be included at the end of the Abstract.
For randomised controlled trials (RCTs), abstracts should include the checklist items set out in the CONSORT guidelines.
If data have been deposited in a public repository authors should include the dataset name and repository name and number at the end of the Abstract.
Graphical abstracts
Authors whose papers (reviews, original articles and short communications) survive the initial round of peer-review will be asked to supply a graphical abstract with their revised version. Please note that this does not need to be supplied at first submission.
The aim of a graphical abstract is to give readers a visual summary of the main message of the paper. Graphical abstracts will appear in the html version of a paper (not the pdf) and will also be used in Diabetologia’s electronic tables of contents (eTOC) alerts, on our website and on twitter. For examples, please click here.
In line with Cell Press’s guidelines for graphical abstracts, please ensure that your graphical abstract meets the following criteria:
Content
The graphical abstract should:
- Have a clear start and end, ‘reading’ from top‐to‐bottom or left‐to‐right
- Provide a visual indication of the biological context of the results depicted (subcellular location, tissue or cell type, species, etc)
- Emphasise the new findings from the current paper without including excess details from previous literature
- Avoid the inclusion of speculative features (unless the speculative nature can be made apparent visually)
- Limit use of data items (maximum one graph)
- Not include abbreviations unless these are defined within the graphical abstract
- Not be a repetition of one of the figures already included in the paper, although authors may wish to develop one of the figures, simplify a schematic or incorporate elements of several figures
The graphical abstract should also:
- Use simple labels
- Use text sparingly
- Highlight one process or make one point clear
- Be free of distracting and cluttering elements
Permission/acknowledgement
Please note that it is the authors’ responsibility to check whether permission or an acknowledgement is required for any part of the graphical abstract and to include details of any acknowledgements on the title page of the manuscript. We can then insert the appropriate wording on the graphical abstract for you. Images from illustration providers (e.g. Servier Medical Art, Wikimedia Commons or Biorender) may need to be accompanied by an acknowledgement of the source and/or may require payment for the right to use the image or a licence to publish. It is important to check the original source of images found on other websites as licences may be required—this can often be checked via google images. Figures/tables taken or adapted from other published sources may require permission from the publisher and/or author.
The following sources have images and templates that are free to reuse, and there are numerous other similar websites where you may find suitable images; however, it is important to check licensing terms for each image, as these may differ between images from the same source:
Format
- TIFF (at least 300 dpi), Powerpoint, EPS, PDF
- Sans serif font e.g. Helvetica or Arial. Please use the same font for all figure labels
- Font size: 12–16 points
- The graphical abstract should be one single panel
- Avoid heavily saturated, primary colours. You may wish to consult the Diabetologia colour palette (https://diabetologia-journal.org/wp-content/uploads/2018/08/Full-colour-palette-for-schematics-and-diagrams.ai.pdf)
Twitter/X
We are committed to making your research as widely accessible as possible and will post on Twitter/X about accepted papers from our account (@DiabetologiaJnl). Please include a post, along with institutional and personal Twitter/X handles and relevant hashtags (maximum of 250 characters, including spaces), on the title page of your article. Your post can be based on the title of your paper or the main finding(s), and should be worded appropriately to be issued from @DiabetologiaJnl. We will include your graphical abstract with the post.
Keywords
Up to ten keywords should be provided, in alphabetical order, at the end of the Abstract.
Abbreviations
Please use abbreviations only when necessary and define them in a separate list, in alphabetical order, given after the keywords. Abbreviations should not normally appear in the title or Abstract. Do not abbreviate type 1 diabetes / type 2 diabetes (only permissible in tables and figures when the abbreviation should be explained in the legend or footnote). Endocrine pancreatic cells should be designated as beta cells (not ß cells or b cells), alpha cells, delta cells and pancreatic polypeptide cells. Please note we have a list of accepted abbreviations that do not need to be defined in the text or included in the abbreviations list.
Inclusive language
Diabetologia encourages the use of inclusive language which is free from bias, stereotyping, sexism, racism and negative connotation.
Research in Context
For submissions of original articles, short communications, systematic reviews and meta analyses, please insert a ‘Research in context’ summary (<200 words) below the abstract to include the following questions and your responses:
- What is already known about this subject? (maximum of 3 bullet points)
- What is the key question? (one bullet point only; formatted as a question)
- What are the new findings? (maximum of 3 bullet points)
- How might this impact on clinical practice in the foreseeable future? (one bullet point only)
Introduction
The Introduction should contain a clear statement of the aim and novelty of the study. It should include neither results nor conclusions.
Methods
Sufficient information should be given to allow a knowledgeable reader to understand what was done, and how, and to assess the biological relevance of the study and the reliability and validity of the findings.
The methods must be detailed enough that others with access to the data would be able to reproduce the results. If results in a paper published in Diabetologia cannot be reproduced the journal will consider publication of refutations of that paper.
If an organisation was paid or otherwise contracted to help conduct the research (e.g. data collection and management) then this should be detailed in the methods.
Clinical trials mentioned in the text The International Committee of Medical Journal Editors (ICMJE) recommends that, where trials are mentioned, for example in secondary analyses or meta-analyses, the trial registration number should be included at first mention of the trial in the manuscript.
Selection and description of observational and experimental participants Clearly describe the selection of observational and experimental participants (including control participants). Include eligibility and exclusion criteria and a description of the source population, commenting on how representative the study sample is of the larger population of interest. Detailed descriptions should be provided of the participants’ clinical characteristics (including the type of diabetes e.g. type 1 diabetes, type 2 diabetes, GDM) upon which individuals were classified. Ensure correct use of the terms sex (when reporting biological factors) and gender (identity, psychosocial or cultural factors) and, unless inappropriate, report the sex and/or gender of study participants. If the study involved an exclusive population, for example in only one sex, justify why, except in obvious cases (e.g. GDM). Explain how you determined race or ethnicity and justify their relevance. In cases where race or ethnicity data were not collected, explain why. Race and ethnicity are social and not biological constructs, and so results should be interpreted in that context.
Informed consent and ethics committee approval A paper describing experimental work in humans must (1) indicate that informed consent has been obtained from patients where appropriate, (2) include a statement that the responsible ethics committee (institutional review board) has given approval, and/or indicate that the reported investigations have been carried out in accordance with the principles of the Declaration of Helsinki as revised in 2008. For studies in children/minors, where informed consent has been provided by the parent or guardian, assent should also be given directly by the child, where practical. Please mention in your paper whether this has been sought and obtained. Do not use participant names, initials or hospital numbers, especially in illustrative material. For studies involving human embryos, gametes and stem cells, please see Springer’s policy guidelines and ensure that the manuscript includes an ethics statement identifying the institutional and/or national research ethics committee approving the experiments and describing any relevant details. Authors should confirm that informed consent was obtained from all recipients and/or donors of cells or tissues, where necessary, and describe the conditions of donation of materials for research, such as human embryos or gametes. Uniform requirements should be followed for ethical standards.
Matching Please give matching criteria for continuous variables, e.g. age +/- 2 years, BMI +/- 1 kg/m2. If these variables were not actually matched and were simply similar between the three groups, then please make this clear.
Systematic reviews and umbrella reviews Diabetologia’s policy is that any paper that includes a systematic review or umbrella review must be registered with one of the established agencies that provide such a service (e.g. PROSPERO, Open Science Framework, Research Registry) and the registration number should be included in the manuscript.
Preclinical studies Diabetologia endorses the NIH guidelines for reporting preclinical research. In line with this, please include the following information in your article:
- Statistics Include a sentence to state how your data were expressed e.g. ‘Data are expressed as means (SEM)’. Please provide details of statistical tests used in your analysis.
- Replicates State how often each experiment was performed and whether the results were substantiated by repetition under a range of conditions.
- Randomisation State whether the samples were randomised and specify the method of randomisation.
- Blinding State whether experimenters were blind to group assignment and outcome assessment.
- Inclusion and exclusion criteria State the criteria used for exclusion of any data, samples or animals. Include any similar experimental results that were omitted from the reporting for any reason, particularly if the results do not support the main findings of the study. Describe any outcomes or conditions that were measured or used and are not reported in the results section.
- Animal studies The source (supplier’s name and country), species, strain, international strain nomenclature, sex, genetic background and age of animals should be given, as well as details of housing and husbandry. The Editorial Board will pay particular attention to the ethical aspects of animal studies. Reports of animal studies must state that the study was approved by the local ethics committee or that the study was conducted in accordance with the Guide for the care and use of laboratory animals, Eighth edition (2011). The editors reserve the right to reject manuscripts that do not comply with the above-mentioned requirements. HOMA is not acceptable for use in animal models since it is not a validated method in animals. The authors will be held responsible for false statements or for failure to fulfil the above-mentioned requirements. Animals should be described as being killed rather than sacrificed.
- Studies involving cell lines (Human or animal cell lines, not donor cells) Report the source, authentication and mycoplasma contamination status of cell lines used.
- Antibodies Report the source, characteristics and dilutions of antibodies and how they were validated.
Key resources Research Resource Identifiers (RRIDs) should be provided for resources used in the study, such as novel genetic tools, transgenic animals, antibodies or other non-standard reagents. See the RIID Portal for more information about RIIDs: https://scicrunch.org/resources
Equipment Manufacturer, city, state (if applicable) and country must be given.
Chemical substances Chemical substances must be properly identified. Except for standard laboratory chemicals, the source of supply (supplier’s name and country) must be given. Drugs must be identified by the generic or official name wherever possible. Proprietary names should be avoided.
Buffers and incubation media Compositions of incubation media should be described, or a reference supplied, together with the pH. Concentrations of solutions should be described in molar terms (mol/l and subunits thereof), equivalents, or percentage weight/volume (wt/vol.) or weight/weight (wt/wt). Mass concentration should be expressed as g/l (or subunits thereof – mg/l or µg/l). It should always be made clear whether concentrations in a mixture are final concentrations or those of solutions added.
qPCR The journal supports the use of the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines for the reporting of quantitative real-time PCR experiments.
Genes and proteins Italic characters should be used for gene symbols including genotypes, alleles, mRNA etc. Upright font and upper case letters are generally used for protein abbreviations. For example:
Human gene: XYZ
Rat/mouse gene: Xyz
Protein (any species): XYZ
For further details see the guidelines recommended by the Human Gene Nomenclature Committee, or by the Jackson Laboratory.Radioisotopes Isotopically labelled chemicals should be identified by the atomic number and symbol of the isotope and its location in the molecule. The specific activity of the starting material should be given in terms of becquerels (Bq: disintegrations/second) per molar weight.
Bioassays and radioimmunoassays An appropriate measure of the precision in terms of standard deviation (SD), standard error of the mean (SEM), coefficient of variation or 95% confidence intervals should be given. Reference to the original technique should be included.
Units of measurement
- Units should conform to the SI convention, except for blood pressure (which should be expressed in mmHg) and haemoglobin (g/l).
- Gas or pressure values should be given as mmHg with kPa in parentheses or vice versa.
- Where molecular weight is known, the amount of a substance should be expressed in mol or appropriate subunit (mmol etc.). For an SI conversion table see AMA Manual of Style: SI Conversion Table. An exception is made for administered doses of insulin, which can be given in U.
- Energy should be expressed in kJ (values in kcal may be given in parenthesis if desired).
- Results for HbA1c should be presented in mmol/mol with percentage units in parentheses, e.g. HbA1c level was 31 mmol/mol (5.0%), in the main text and electronic supplementary material ESM. In tables, please include HbA1c values expressed in mmol/mol in a separate row above the % values. With the exception of regression analyses (where units should be reported in mmol/mol), please dual report HbA1c values for all analyses. HbA1c values should be presented in mmol/mol in figures. If referring to previously reported results (e.g. in the Discussion section of your paper), please use mmol/mol.
- Please specify whether % concentrations are by weight or by volume (e.g. wt/vol., wt/wt)
- The solidus may be used in a unit but not more than once (e.g. mmol/l is acceptable, but ml/min/kg is not acceptable and should be replaced with ml min–1 kg–1).
Statistical analyses Describe statistical methods in sufficient detail to enable a knowledgeable reader with access to the original data to verify the reported results. Computer software packages that are used for anything other than widely known standard statistical procedures should be identified by name or acronym and by author or organisation of origin. If t tests were used it should be stated whether these were paired or unpaired. Reference for statistical methods should preferably be to standard works (with pages stated) rather than to papers in which designs or methods were originally reported. When variability is expressed in terms of the SEM or SD, the number of observations (n) must also be given (please provide exact n values, rather than ranges, e.g. n=3–6). When possible, quantify findings and present them with appropriate indicators of measurement error or uncertainty (such as confidence intervals). Both the sample size and statistical significance should be predefined. Details of statistical outcomes should be given, such as estimated effect size, precision and significance (e.g. p values). If necessary, professional statistical advice will be sought by the Editor-in-Chief.
Use of the terms ‘trend’ or ‘tended to’ is not permitted when discussing data that do not reach the threshold for significance. Instead, it should be stated that the difference reported is not significant and statistical values (e.g. p values) should be provided. Note that this does not apply to studies in which ptrend is reported.
Footnotes
Footnotes should not be used in the main text.
Results
The results should be stated concisely without discussion and should not normally contain any references. The same data should not be presented in figures and tables. Do not repeat all the data that are set out in the tables or figures in the text; emphasise or summarise only important observations.
Discussion
The Discussion should deal with the interpretation of the results and not recapitulate them. We encourage authors to write their Discussion in a structured way, as follows:
- Statement of principal findings;
- Strengths and weaknesses of the study;
- Strengths and weaknesses in relation to other studies, discussing important differences in results;
- Meaning of the study: possible explanations and implications for clinicians and policymakers;
- Unanswered questions and future research.
Acknowledgements
Acknowledgements should be as brief as possible. Any editorial assistance should be acknowledged. For individuals thanked in this section, or acknowledged elsewhere in the text, please provide names (initials and surname) and affiliations.
Data availability
Please include a statement of data availability. This should include information on where data supporting the results reported in the article can be found (including, where applicable, hyperlinks to publicly archived datasets analysed or generated during the study), or whether the data are available on request from the authors or if no data are available. Springer Nature provides examples of data availability statements.
Research data policy Submission to Diabetologia implies that materials described in the manuscript, including all relevant raw data, will be freely available to any researcher wishing to use them for non-commercial purposes, as long as participant confidentiality is not breached.
Diabetologia strongly encourages authors to make available to readers all datasets on which the conclusions of the paper rely. We encourage authors to ensure that their datasets are either deposited in publicly available repositories (where available and appropriate) or presented in the main manuscript or additional supplementary files whenever possible. Please see Springer Nature’s information on recommended repositories. General repositories – for all types of research data – such as figshare and Dryad may be used where appropriate.
Where a widely established research community expectation for data archiving in public repositories exists, submission to a community-endorsed, public repository is mandatory. Persistent identifiers (such as DOIs and accession numbers) for relevant datasets must be provided in the paper.
For the following types of data set, submission to a community-endorsed, public repository is mandatory:
Mandatory deposition Suitable repositories Protein sequences Uniprot DNA and RNA sequences Genbank
DNA DataBank of Japan (DDBJ)
EMBL Nucleotide Sequence Database (ENA)DNA and RNA sequencing data NCBI Trace Archive
NCBI Sequence Read Archive (SRA)Genetic polymorphisms dbSNP
dbVar
European Variation Archive (EVA)Linked genotype and phenotype data dbGAP
The European Genome-phenome Archive (EGA)Macromolecular structure Worldwide Protein Data Bank (wwPDB)
Biological Magnetic Resonance Data Bank (BMRB)
Electron Microscopy Data Bank (EMDB)Microarray data (must be MIAME compliant) Gene Expression Omnibus (GEO)
ArrayExpressCrystallographic data for small molecules Cambridge Structural Database Datasets that are assigned digital object identifiers (DOIs) by a data repository may be cited in the reference list. Data citations should include the minimum information recommended by DataCite: authors, title, publisher (repository name), identifier.
For more information see Research Data Policy Frequently Asked Questions.
Diabetologia requires authors to provide a statement of data availability in their article. Data availability statements should include information on where data supporting the results reported in the article can be found, including, where applicable, hyperlinks to publicly archived datasets analysed or generated during the study. Data availability statements can also indicate whether data are available on request from the authors and where no data are available, if appropriate. Click here for examples of data availability statements, including examples of openly available and restricted access datasets.
Springer Nature provides a research data policy support service for authors and editors. This service provides advice on research data policy compliance and on finding research data repositories but does not advise on specific manuscripts.
Funding
Please include a separate Funding section after your Acknowledgements which details your sources of funding. Any grant support that requires acknowledgement should be mentioned. The names of funding organisations should be written in full.
Where no specific funding was received, please insert the following statement: ‘This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors’
Role of study sponsor or funder The ICMJE uniform requirements for manuscripts submitted to medical journals state that authors should describe the role of the study sponsor or funding source, if any, in study design; collection, analysis, and interpretation of data; writing the report; and any restrictions regarding the submission of the report for publication, e.g. ‘The sponsor/funder provided editorial assistance only.’, ‘The sponsor/funder was involved in study design and data collection only’. If the sponsor/funder had no such involvement, the authors should state ‘The study sponsor/funder was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.’
Authors’ relationships and activities
Authors are responsible for recognising and disclosing relationships and activities that readers could perceive to have influenced, or that give the appearance of potentially influencing, their work. They should acknowledge in this section any industry links and other personal connections.
Contribution statement
The ICMJE uniform requirements for manuscripts submitted to medical journals state that authorship credit should be based on:
- Substantial contributions to conception and design, acquisition of data or analysis and interpretation of data
- Drafting the article or reviewing it critically for important intellectual content
- Final approval of the version to be published.
All three conditions must be met by all authors. Participation solely in the acquisition of funding or data or general supervision of the research group does not constitute authorship.
Please include a statement listing each author’s contribution. Please ensure that this is discussed with your co-authors and agreement is reached prior to manuscript submission. Post-acceptance changes to the author list will not be permitted.
In accordance with the Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals (ICMJE Recommendations 2013), please identify the guarantor(s) at the end of the Contribution Statement. The guarantor accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
References
References to the literature should be credited to the original findings and be in numerical order in the text, the number being given in square brackets on the line, and numbered in the same order at the end of the manuscript. References should not be used by authors, editors or peer-reviewers to promote self-interests. There must be only one reference per number. Reference may only be made to Abstracts published in the current or preceding year. References to material on a preprint server should be included in the reference list using the format below, but should be replaced by the peer-reviewed, published version if this becomes available. Authors should not cite artificial intelligence (AI)-assisted technologies (such as Large Language Models [LLMs], chatbots or image creators as an author and cannot reference AI-generated material as the primary source.
Reference list In accordance with Springer publishing policy, all references should be in the ELSE-Ciba style, as follows:
Articles in journals Names of up to six authors with initials (seven authors or more should be abbreviated to et al after the third author’s name); (year); title of paper in full; abbreviated name of journal (according to the NLM Catalog); volume number (issue number); first and last page numbers (full page range). DOI, e.g. Tanaka Y, Tran PO, Harmon J, Robertson RP (2002) A role for glutathione peroxidase in protecting pancreatic ß cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci U S A 99(19):12363–12368. https://doi.org/10.1073/pnas.192445199
Whole book Names of all authors with initials; (year); title of book; edition; name and city of publisher, e.g. Ekoé JM, Zimmet P, Williams R (2001) The epidemiology of diabetes mellitus. An international perspective. Wiley, Chichester
Chapter from a book Names of all authors with initials; (year); chapter title; In: editors’ names with initials (eds); title of book; volume number; name and city of publisher; pp first and last page numbers, e.g. Hopper JL (2000) Why ‘common environmental effects’ are so uncommon in the literature. In: Spector TD, Snieder H, MacGregor AJ (eds) Advances in twin and sib-pair analysis. Oxford University Press, London, pp 151–165
Letters to the Editor As for articles in journals, e.g. t’Hart LM, Dekker JM, Heine RJ, Maassen JA (2003) Lack of association between gene variants in the ALMSI gene and type 2 diabetes mellitus. Diabetologia 46:1023–1024
Abstracts As for articles in journals, with (Abstract) after the page numbers, e.g. Khalifah RG, Chen Y, Price DL, Booth AA (2002) Mechanism of inhibition of advanced glycation endproducts by Pyridorin™, a novel therapeutic for diabetic complications. Diabetologia 45(Suppl 2): A1222 (Abstract); citations to abstracts is only permissable if they date from the current or preceding year (an exception is made for meta-analyses, systematic reviews and umbrella reviews, which may cite abstracts from previous years)
Material on preprint server This should include ‘(preprint)’ and the version date, as well as the date you accessed the material e.g.: Bar DZ, Atkatsh K, Tavarez U, Erdos MR, Gruenbaum Y, Collins FS (2016). Biotinylation by antibody recognition–a novel method for proximity labeling. BioRxiv 069187 (Preprint). 11 Aug 2016. Available from: https://doi.org/10.1101/069187 (access date); citations to material on preprint servers is only permissable if they date from the current or preceding year (an exception is made for meta-analyses, systematic reviews and umbrella reviews, which may cite preprints from previous years)
Website Authors; (year); title; URL; date accessed, e.g.: Regional Office for the Western Pacific of the World Health Organization, International Association for the Study of Obesity and the International Obesity Task Force (2000) The Asia-Pacific perspective: redefining obesity and its treatment. Available from www.obesityasiapacific.com. Accessed 10 October 2003
Papers quoted as ‘in press’ Authors should provide an electronic version of any manuscripts cited as ‘in press’ when they submit their manuscript to Diabetologia. If an accepted paper contains references to a manuscript ‘in press’, written evidence that the manuscript has been accepted will be requested. Numbered references to personal communications, unpublished data and manuscripts either ‘in preparation’ or ‘submitted for publication’ are unacceptable. If essential, such references may be incorporated in parentheses in the appropriate place in the text, but written consent for publication must be provided.
The references are the responsibility of the authors. They must be written correctly and be rechecked by the authors in the proofs. References to abstracts (only current and preceding year), letters to the editor, congress proceedings, and non-peer-reviewed publications should be kept to a minimum.
EndNote style template A Diabetologia style template is available for EndNote and can be downloaded here. Instructions for installing styles are available on the EndNote styles page: https://endnote.com/downloads/styles/.
Mendeley If using Mendeley, please select the Diabetologia style.
Tables
Please ensure that all tables have been created using the ‘insert table’ function of Word and that each cell contains a single value (± SD/SEM/superscript letters, if applicable) only. Do not merge cells. Subgroups should be presented in separate rows; please use indentation to indicate different sections. Tables should be numbered with Arabic numerals; multi-part tables are not permitted. Each table should have a short informative heading which is self-explanatory without reference to the text. Footnotes should be kept to a minimum. The method used should be described in the text of the Methods section and not in a footnote. Only conditions specific to the particular experiment should be stated. The units in which the results are expressed should be given at the head of each column or after each variable [either separated by a comma or in parentheses e.g. Men, n (%); Age, years]. Internal column headings indicating a change of units are not permitted within tables. Superscripts ‘a’, ‘b’, ‘c’ should be used for footnotes (asterisks are permitted for p values).
Large tables summarising information from different sources, for example studies analysed in reviews, systematic reviews and meta-analyses, can present problems in terms of size and layout, so it is important to consider how best to set them out before submission; in particular, please consider whether you have ordered the entries in your table in the most appropriate way, for example alphabetically, in date order, by category, in date order within categories etc. Large amounts of additional information can be submitted for publication electronically as supplementary material.
There is no restriction to the number of tables permitted in an original article.
Figures
It is the responsibility of the authors to ensure that graphs accurately reflect the data they represent. The addition of error bars etc by hand is unacceptable. Graphs may occasionally be scrutinised for evidence of inappropriate manipulation.
Figures should be provided in separate files (and not embedded in the main document). Figure legends should be included at the end of your article. See the recent issues of Diabetologia for general style and layout.
Diabetologia offers its authors free use of colour in figures. For graphs and flow diagrams, please select colours from the Diabetologia colour palette. The extended colour palette may be used for schematics and diagrams only.
Our artwork guidelines should be referred to and followed where possible, and further advice can be obtained from the Editorial Office if needed. The guidelines are given to ensure that figures can be published as clearly as possible, and following our style will mean fewer changes during copy-editing and typesetting.
Data integrity Authors of papers containing cropped gels/immunoblots (including supplementary images) are required to upload, at the point of submission, a separate PDF or PowerPoint file (distinct from any other supplementary material) that displays the entire unedited gel/image. Authors should indicate clearly which bands were used in which figure/figure part. All potentially accepted manuscripts will undergo image forensics analysis as part of the review process.
Image manipulation Diabetologia has adopted the following statement developed by Cell Press as its policy on the manipulation of digital images:
Authors should make every attempt to reduce the amount of post-acquisition processing of data. Some degree of processing may be unavoidable in certain instances and is permitted provided that the final data accurately reflect the original. In the case of image processing, alterations must be applied to the entire image (e.g. brightness, contrast, colour balance). In rare instances where this is not possible (e.g. alterations to a single colour channel on a microscopy image), any alterations must be clearly stated in the figure legend and in the methods section. Groupings and consolidation of data (e.g. cropping of images or removal of lanes from gels and blots) must be made apparent by the arrangement of figures (e.g. dividing lines) and should be explicitly indicated in the text of the figure legend. Data comparisons should only be made from comparative experiments, and individual data should not be utilised across multiple figures. In cases where data are used multiple times (e.g. multiple experiments were performed simultaneously with a single control experiment), this must be clearly stated within each figure legend. In the event that it is deemed necessary for proper evaluation of the manuscript, authors will be required to make the original unprocessed data available to the editor.
Image editing software and investigative techniques will be used to screen images in potentially acceptable papers to identify any manipulation. Any untoward manipulation will be investigated by the Scientific Integrity Panel following guidelines set out by the Committee on Publication Ethics (COPE; http://publicationethics.org/).
Permissions If the figure has previously been published, please see our section on Permissions.
What happens to figures if your paper is accepted? If your paper is accepted or provisionally accepted, you may be asked to make certain changes to your figures to comply with journal style prior to copy-editing. In addition, we may make further changes to figures at the copy-editing stage, either by editing them ourselves, marking up the images to be changed by our typesetters, or we may request that you make additional amendments and supply replacement figures. This process will help to ensure that your data are clear and accessible to readers, and that images are consistent between different articles in Diabetologia. You will be given the opportunity to review the figures, with the rest of the article, at the proof stage. It is important to take care when checking the proofs as changes cannot be made once the article has been published online.
Legends for figures
Figure legends should be concise and self explanatory and contain enough information to identify the figures and enable them to stand as a separate entity from the text. However, legends should not give methodological detail (this should be included in the Methods) and repetition of information in the main text should be avoided. Headings and statistical analysis should be included in the legend rather than the figure. Figure legends (but not ESM figure legends) should be provided at the end of the main text, after the references, not in the figure files.
There is no restriction to the number of figures permitted in an original article.
- Artwork guidelines
-
We expect artwork to be clear and informative so that, as far as possible, figures can be understood as stand-alone images, when read alongside the figure legend, as well as in the context of the paper as a whole.
Graphs and diagrams
Please ensure it is clear in the figure or main figure legend how the data are presented.
To keep figures uncluttered please omit headings, captions and statistical values; this information should be included in the main figure legend instead.
Please use standard statistical notation in figures (*p<0.05, **p<0.01, ***p<0.001); usual order for symbols for different comparisons is: *, †, ‡, §, ¶; please don’t use # or $ signs.
Please provide exact n values for numbers of samples for each figure part, rather than, for example, n=3–6, unless the number of data points can easily be read from the graph (see ‘Bar charts’ below). For complex experiments including variable numbers of samples and replicates, consider including this information in a table under the x-axis.

Data should not extend beyond the upper or lower values shown on the axis.
Please avoid borders or frames around graphs. Do not use gridlines.
Please use a weight of approximately 0.75 pt (3/4 pt) for axis lines and lines within graphs. Axis lines should be black.
Diabetologia encourages use of colour in graphs; please select colours from the Diabetologia colour palette. If you need to use a patterned fill for bar charts, widely spaced horizontal or diagonal stripes are most effective.
Symbols in graphs should be clear and easy to distinguish from one another. If there are only two symbols used, it is clearest to use two different colour fills e.g. one black symbol and one white. Otherwise use different colours or symbols, for example orange, red, blue, green squares; or black squares, white squares, black triangles, white triangles etc. For scatter diagrams and line graphs, solid symbols are preferred.
Bar charts
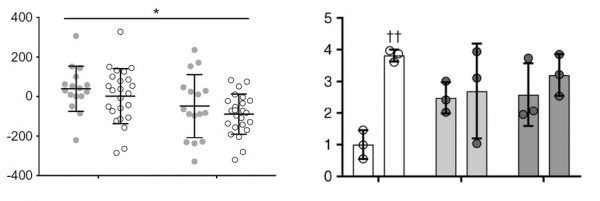
Present data as individual points rather than as simple bar charts. This can either be in the form of a bar chart with individual data points shown clearly on the graph, or a column scatter graph, as shown in the examples below. Different symbols can be used for different samples or treatments, if appropriate. Please use lines to show the means and error bars, and make sure these can be read clearly.
If bar charts are required (e.g. to avoid crowding where there are a large number of samples), fills should be easy to distinguish.
Box and whisker plots
Box and whisker plots can either be presented with individual data points (similarly to bar charts), or without; if you use the latter option, please ensure the number of measurements is clear in the figure legend (n=XXX).
Please include details of how the data are presented in the figure legend, particularly the ‘whiskers’, which may indicate different things, depending on the type of box plot, e.g. minimum and maximum; 1 SD above and below the mean.
Line graphs
Curves should not be drawn beyond experimental points.
Where dashed lines are used, dashes should be sufficiently large and spaced to be seen clearly when figures are reduced.
Forest plots
If your forest plot presents data from multiple previous studies, please include:
- the name of the study or first author’s name followed by et al (give names of both authors if there are only two authors);
- year of publication (where appropriate);
- the reference citation number [in square brackets].
If possible, please provide forest plots as PowerPoint files in which the text is editable, or as EPS, PDF or Adobe Illustrator vector files. If this is not possible, please supply them as TIFF files at a resolution of 1200 dpi.

Flow charts

Flow charts should be supplied as editable Word or PowerPoint documents, if possible, or as EPS, PDF or Adobe Illustrator vector files.
For flow charts, colours should be selected from the Diabetologia colour palette. Please avoid use of bold font and use a weight of 0.5–1.5 pt for lines, borders and arrows.
Please check that values add up where appropriate.
Schematic diagrams
Schematic diagrams should be provided as editable PowerPoint files if possible. If other file types are supplied, authors may be asked to make stylistic changes themselves.
For schematic diagrams, colours should be selected from the extended colour palette. Different styles can be used for boxes and shapes but, similarly to flow charts, please avoid bold and use a weight of 0.5–1.5 pt for lines, borders and arrows.
For reviews, schematic and illustrative figures may be re-drawn by our medical illustrator, who will liaise with the authors.

Photographs, micrographs and gels
Photographs and images of gels, micrographs or photographs should be inserted into figures at a resolution of at least 300 dpi.
Labels must be large enough to be read easily after reduction for publication and should contrast clearly against the background. If possible, please insert labels using a vector package such as Adobe Illustrator or PowerPoint (see ‘Labels and wording in figures’ below). For example, images could be checked and cropped in Photoshop, and then imported into Adobe Illustrator or PowerPoint for any text and symbols to be added.
Scale should be indicated in micrographs using an internal reference marker (scale bar) on the photograph itself (as a horizontal line at least 1 mm thick after reduction). The scale should be given in the legend (e.g. ‘scale bars, 20 nm’), rather than on the image itself.
If photographs of patients/individuals are used, the individual should not be identifiable or the picture must be accompanied by written permission to use the figure.
Colour images
There is no charge for use of colour in figures. For graphs and flow diagrams, please select colours from the Diabetologia colour palette. The extended colour palette may be used fo
 r schematics and diagrams only. Please see ‘Graphs and diagrams’ section above for more information.
r schematics and diagrams only. Please see ‘Graphs and diagrams’ section above for more information.Multipart figures
Figure parts should not be supplied as individual files; please incorporate them into a composite figure as you would like them to appear in the final version of the paper. When deciding on the layout, please consider how it will look in print, and how clear it will be to readers. Try to minimise white space. Figure layout will sometimes be changed by the Editorial Office to make sure that space is optimised.
The composite figure should be an appropriate size for publication. Generally figures will be set in a single column (8.4 cm) or less with the legend below, or in 1.5 columns (12.9 cm) with the legend to one side. These sizes may be changed slightly by the Editorial Office to ensure consistency. Larger composite figures will be set at up to full page width (17.4 cm). The maximum page height is 23.5 cm.
Avoid referring to ‘upper’, ‘lower’, ‘left’ and ‘right’ panels—generally it is clearer for these panels to be given their own individual figure part label and described separately in the main figure legend. In line with this, each figure part should usually have its own part label (a, b, c etc.), although exceptions can be made, for example: gel or blot with associated quantification graphs; line graph with associated AUC graph; composites of micrographs (including associated quantification graphs).
Separate figure parts should be labelled with lower case letters and should be in alphabetical order (either horizontally or vertically).

Separate parts should be sized so that they are consistent across the figure; similar graphs should be the same size and font sizes should be the same throughout the figure, as far as possible.
Each individual graph should usually have its own x- and y-axis labels, even where these are repeated along rows or columns of graphs.
If possible, use editable vector fonts for all labels and symbols, by using a vector program such as Adobe Illustrator or PowerPoint to put together your figures. See ‘Labels and wording in figures’ below for more information.
Photographic images can be prepared in Photoshop and then inserted into Adobe Illustrator or PowerPoint for layout and labelling.
Where images are inserted into other programs, such as PowerPoint, Adobe Illustrator or Photoshop, please ensure the image is inserted at the correct resolution. This can generally be done by using the ‘insert’ function, provided the image file to be inserted is at the right resolution. Cutting and pasting may result in a low-resolution image.
Labels and wording in figures
All figures should be numbered using Arabic numerals (Fig. 1, Fig. 2 etc.) and figure parts should be labelled with lower case letters (Fig. 1a, b etc.).
If possible, please use editable vector fonts for all labelling and lettering in your figures (for example with a program such as Adobe Illustrator or PowerPoint); this ensures the text is clear and allows the typesetters to make any necessary style changes.
Use a sans serif font for numbers and lettering: Helvetica if available, otherwise Arial. Please use the same font for all figure labels in your manuscript.
Courier font can be used for sequence alignments.
Font size should be selected so that it is easily readable (final size 2–3 mm, 8–12 pt) when the figure is reduced for publication. This means that the font may need to be considerably larger when preparing the figure, so that it will be 8–12 pt once the image has been reduced.
Please use a consistent font size for x– and y-axis labels and across different figure parts.
Labels should be in sentence case (the first letter of the first word upper case and the rest of the label lower case, except for proper names, abbreviations etc.), e.g. ‘Fasting plasma glucose…’.
Numbers on the x– and y-axes should read horizontally wherever possible.
If the axis shows a measurement, please ensure that units are included in parentheses at the end of the axis label, e.g. ‘Plasma glucose (mmol/l)’.
Please use SI units for all measurements in figures and in the main text. For an SI conversion table see the AMA Manual of Style conversion calculator.
Please ensure that the terminology, formatting and spelling in figure labels is the same as in the text. For example, where gene symbols are included, please use gene formatting (italic), and please also use UK English spelling.
Embedding fonts
For figures generated in Adobe Illustrator or Microsoft Office, or for other vector files, please embed the fonts when you save the document. This ensures that any non-standard fonts used will remain part of the file.
Adobe Illustrator If you save as AI format, the subset of the font used in the image will be embedded automatically. If you save in EPS format, please tick the ‘Embed fonts (for other applications)’ box when saving.
MS Office For Office 2013 and earlier: when you click ‘Save as’, the tools menu (next to the ‘Save’ option in the dialogue box) gives you the option of embedding fonts; For Office 2016 onward: first, click on the File tab and then select ‘Options’. In the dialog box, select ‘Save’ and check the ‘Embed fonts in the file’ box at the bottom of the page.
- Figure format
-
If your paper is accepted, you will be asked to supply original vector files if possible, or high-resolution TIFF files. Graphs and diagrams are best provided as EPS vector files, or in another vector format such as PowerPoint, Excel or PDF, so that lines and text will remain clear when the figure is resized for publication. Vector-based images are made up of lines rather than pixels. Because they are not comprised of a specific number of dots, they can be scaled to a larger size without loss of image quality. Resolution, which is important for non-vector files, is not an issue for vector files because of this scalability. Supplying vector files in one of the formats shown below will ensure that the final print quality of the images is good.
Because of file size limitations you may wish to upload lower resolution versions onto ScholarOne Manuscripts for review purposes when submitting your paper. Higher resolution figures will be requested if your paper undergoes revision or is accepted.
The following file types are preferred:
Vector
- EPS
- Adobe Illustrator (AI)
- PowerPoint
- Excel
- Word (generally acceptable only for flow charts/schematics)
Non-vector
- TIFF
- PNG
Notes on particular software packages
Stata and GraphPad For information on converting figures created in Stata and GraphPad to EPS vector files, please see our detailed instructions.
SPSS Graphs created in SPSS cannot always be saved with sufficient resolution for publication, although we have been advised by SPSS/IBM that exporting as a PDF is likely to give the best results. Depending on the clearness of your images, you may need to replot data from this program in an alternative program.
Microsoft Office The resolution of TIFF files saved from Microsoft Office is not usually high enough for publication, so please send the original Microsoft files (PowerPoint. Excel, Word).
TIFF files
Resolution is important for these non-vector files because the image will be fuzzy and pixelated if the resolution is not high enough.
For non-vector files, please supply TIFFs saved at a resolution of 1200 dpi for black and white graphs, and 600 dpi for graphs containing grey or coloured fills, gels, blots, micrographs and composite images (a mixture of line graphs and other images).
When increasing the resolution of figures, please avoid resampling (for example in the ‘Image size’ function in Adobe Photoshop), as resampling will result in the artificial addition of pixels (essentially adding data by guesswork), which may lead to inaccurate or blurry Images.
TIFF files created in Photoshop or similar artwork software should not be flattened.
When saving graphs and line art as TIFF files, select ‘none’ for compression, if possible; for halftones use LZW compression.
Colour images should be encoded as RGB (8 bits per channel) rather than CMYK. For graphs and flow diagrams, please select colours from the Diabetologia colour palette. The extended colour palette may be used for schematics and diagrams only.
- Videos
-
Manuscripts containing video material can be published as multimedia manuscripts, and the videos can be embedded in the HTML version of the article, although not in the PDF. The video files would also be included as electronic supplementary material (ESM) so that they are accessible to people downloading the PDF version. This is an alternative to publishing the video files as ESM only.
Up to three videos per manuscript can be embedded, with the following file requirements:
- Aspect ratio: 16:9 or 4:3 (please note that this needs to be exact)
- Maximum file size 25 GB
- Minimum video duration: 1 s
- Supported file formats: avi, wmv, mp4, mov, m2p, mp2, mpg, mpeg, flv, mxf, mts, m4v, 3gp
- Video files should not contain anything that flashes more than three times per second (so that users prone to seizures caused by such effects are not put at risk)
If you wish your video files to be included in this way, please ensure that these requirements are met, provide a video title/caption, and indicate where in the manuscript the video files should be embedded.
- Electronic supplementary material (ESM)
-
ESM can be used in conjunction with full-length papers and short communications.
Additional information (e.g. gene sequences) can be submitted for publication electronically as supplementary material provided that it is not essential for a basic understanding of the main paper. You should include references to the supplementary material at appropriate places in your article; these will become hyperlinks to the ESM in the electronic version of article.
Please supply your ESM as a single pdf file, with parts arranged in this order: ESM text (methods and results), tables, figures (with figure legends underneath).
All units in the ESM should be SI. For an SI conversion table see the AMA Manual of Style conversion calculator.
ESM will be peer-reviewed but will not undergo any copyediting and will be published online exactly as supplied by the author.
ESM References If some or all of the references in the ESM do not appear in the main text, please prepare a separate reference list for papers cited in the ESM, numbered [1], [2], [3] etc. This list should be inserted directly underneath the relevant section of ESM text/table/figure, and each ESM will need its own list. Note that references that appear both in the main text and ESM might have two different reference numbers.
ESM Methods The main text should present sufficient information to allow a knowledgeable reader to understand what was done, why, and how, and to assess the biological relevance of the study and the reliability and validity of the findings. There should be enough details in the main text Methods section so that readers do not need to look at the ESM to understand the procedures/protocols used. Sufficient information should be given to allow the experiment to be repeated but this level of detail may be given in the supplementary material if space does not permit inclusion in the main text.
There are two options for giving experimental details in the supplementary material:
- Describe the majority of the methods in the main text and refer readers to the ESM for details of a specific technique. In this case there must be a call out in the main text to each subheading in the ESM. Please see here for an example of a paper with ESM methods.
- If the experimental techniques are complex we suggest that you describe the methods used in brief in the main text and then give detailed methodology for readers who want to repeat the experiment in the ESM, inserting a note to this effect in the main text. Readers should be able to read the ESM methods without referring back to the main text.
Please ensure that in the ESM, the methods are presented in the same order they are mentioned in the main text. If possible, please use the same subheadings in both the ESM and main text.
ESM Results As with ESM Methods, please ensure that ESM Results are presented in the same order they are mentioned in the main text. If possible, please use the same subheadings in both the ESM and main text.
ESM Tables Please note that we can publish spreadsheets in Excel format as ESM tables if necessary.
Avoid duplicating data from repository Please note that datasets published on your own servers or on publicly archived data repositories should not be included with the ESM, and should be cited and numbered separately from the ESM documents, to avoid duplication. The URL to access these documents should be provided in the data availability statement.
- Supplements
-
Diabetologia does not publish supplements other than the Abstract volume of the annual EASD meeting.
- Revised manuscripts
-
If you are asked to revise your manuscript you will be expected to provide a covering letter that responds in detail to each point raised by reviewers or editors, and to indicate, using a different colour font, all changes and new material in your paper, ensuring that such changes will be clear if referees print your manuscript in black and white (do not use the ‘track changes’ mode of Word). If a manuscript returned to the authors for revision is not returned to the Editorial Office within the stipulated time-period (usually 4 weeks), it may be treated as a new manuscript.
When you submit your revised manuscript please send a completed author disclosure form to the Editorial Office by email (diabetologia-j@bristol.ac.uk) or fax (+44 (0)117 4147887). Please note that every author needs to sign a separate form (i.e. there should be one form per author) and that we can only accept handwritten (not typed) signatures.
- Resubmitted manuscripts
-
If your manuscript was rejected with an invitation to resubmit as a new paper, there is no time limit for submitting your new manuscript as extensive reworking/new experiments may be required. This is in contrast to papers that are returned for revision (see above). If you choose to resubmit your manuscript, you will be expected to include a point-by-point response to the referees’ comments in a covering letter. You will be expected to indicate, using a different colour font, all changes and new material in your paper, ensuring that such changes will be clear if referees print your manuscript in black and white (do not use the ‘track changes’ mode of Word). Please be aware that we might ask new referees to review your resubmitted manuscript.
- Proofs
-
Once your paper has been sent for typesetting, the corresponding author will receive an email from the publisher giving the options of (i) transferring copyright to Springer or (ii) retaining copyright by ordering Open Choice (see next section). Once this copyright transfer process has been completed, a further email giving access to an electronic proof and ESM (URL) is sent to the corresponding author.
Note that once an article has been published online, mistakes can only be corrected by publishing an erratum, so corresponding authors are strongly advised to circulate proofs among all their co-authors so that all those who have contributed to the paper have the opportunity to check carefully that the data are correct. Only essential corrections to typographical and data errors should be made at this stage; stylistic issues should have been dealt with during copyediting.
If any changes are required to electronic supplementary material (ESM), the corresponding author should include a replacement of the ESM pdf with the proof corrections, as ESM is not edited by the Editorial Office.
The proof corrections should normally be returned within 48 h but please do contact the Editorial Office for an extension if more time is needed to allow for thorough checking.
- Errata and Retractions
-
Requests for errata or retractions should be sent to the Editorial Office (diabetologia-j@bristol.ac.uk). Depending on the nature of the request, the Editorial Office may consult with members of the Editorial Board or Diabetologia’s Scientific Integrity Panel.
Errata
Errata refer to errors introduced into the article by the author(s), editorial office or the publisher. Note that once an article has been published online, mistakes can be corrected only by publishing an erratum. Diabetologia will publish errata to communicate necessary corrections to such errors. Once published, the erratum will be linked to the original paper, but neither the print or electronic versions of the original paper will be amended. Errata will appear in the table of contents and are indexed in public databases such as PubMed.
Retractions
Requests for retractions may be referred to Diabetologia’s Scientifc Integrity Panel. Diabetologia follows the guidelines set out by COPE and will consider retracting a publication if:
- There is clear evidence that the findings are unreliable, either as a result of misconduct (e.g. data fabrication) or honest error (e.g. miscalculation or experimental error)
- The findings have previously been published elsewhere without proper cross-referencing, permission or justification
- Plagiarism is detected
- The research is unethical
Co-authors listed on the original paper should be made aware of the problems with the paper and the pending retraction request. Notices of retraction will clearly outline the reason for the retraction and at whose request the paper is being retracted. In cases where co-authors disagree, dissenting author(s) will be noted in the text of the retraction note. All retractions must be approved by our publisher, Springer.
- Offprints
-
Authors will receive an electronic offprint (pdf) free of charge and will have the opportunity to purchase additional printed offprints.
- Permissions
-
Figures/tables taken from previous publications must be accompanied by either a statement (e.g. from RightsLink) giving permission to Diabetologia for reproduction in print and electronic formats, or details of the Creative Commons Attribution (CC BY) licence (which permits sharing and adaptation of another’s work providing the original source is properly cited). Please note that permission is usually required for publication of an adapted figure/table. It is the responsibility of the submitting author to investigate whether permission is needed and to pay any fees associated with obtaining permission.
- Copyright and open access
-
After your paper has been sent for typesetting (i.e. after copy-editing), you will receive an email from our publisher, Springer, asking you to complete a form via which you must transfer copyright to Springer or order Open Choice (thus retaining copyright). For more information on these publishing options, please see Springer’s information on ‘How to publish with us’.
It is the author’s responsibility to check whether there are any open access requirements associated with the funding received. Please see Springer’s funder compliance FAQs for further details.
If you are funded by the NIH or other US Government funding agency (such as CDC), please see Springer’s guidance for NIH-funded authors for further information. Particular rules apply to US Government employees (which includes NIH, CDC and Department of Veterans’ Affairs [VA] employees) and to papers funded through NIH or other government intramural funding: such authors are not permitted to transfer copyright to the publisher. For further information see Springer’s Funder Compliance Policy.